Chemistry 201
advertisement

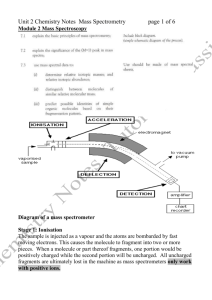
Experiment 8A Mass Spectrometry and Structural Analysis 1. The Mass Selective Detector (MSD). When a neutral substance mass spectrometer, it is bombarded with an electron, which causes the substance to ionize as illustrated in Equation 1. - e + M . + [M] + - 2e Equation 1: Electron Impact Ionization The species [M]+. is both a carbocation and a free radical (has an unpaired electron) and is referred to as a radical cation. The mass of this first formed radical cation is equal to that of the original molecule entering the mass spectrometer and is referred to as the molecular ion. The molecular ion, because it has one fewer electron than is needed to be neutral and stable, will break down (fragment) into smaller and more stable components. These fragments can be neutral, cationic, and/or free radicals. Only those fragments that are cationic will be observed by the detector. A detailed description of the detection process is given in McMurry, Section 12.1-12.4. You should also consult http://www.chem.uic.edu/web1/OCOL-II/WIN/SPEC/MS/MSF.HTM For our purposes in this Worksheet it is important to know the following: • The mass spectrometer detects only positive ions and disperses them on the according to their mass to charge ratio, m/z. • Most ions produced on ionization in the electron beam are uni-positive so numerically, m/z =mass. • The mass of the molecular ion can usually be taken as the molecular weight of the substance. • The MSD can separate ions that have different isotopes of their atoms. • The most intense peak in the spectrum is called the base peak and represents the ion of highest abundance and therefore the highest intensity • Knowledge of the isotope composition of the more common elements is essential to the interpretation of mass spectral data. See Figure 1 2 Mass Spectrometry CHEM 211-07 Figure 1: Table of Isotope Composition of Some Common Elements From this table we can conclude: •• The presence of bromine in a compound leads to an M+2 peak that is nearly equal to that of the molecular ion. •• The presence of chlorine is detected by a M+2 peak about one third the intensity of the molecular ion. •• A single nitrogen in a molecule will give the molecule an odd number for the molecular ion. 2. Fragmentation Patterns in Mass Spectrometry a. Hydrocarbons Figure 2. illustrates the mass spectrum of 2,3-dimethylpentane CHEM 211-07 Mass Spectrometry 3 Figure 2: Mass Spectrum of 2,3-Dimethylpentane 1. Draw a Lewis structure for 2,3-dimethylpentane and number the carbon atoms in the chain. 2. What is the molecular weight in whole number mass units of 2,3-dimethylpentane? 3. Can you detect the molecular ion in the mass spectrum of 2,3-dimethylpentane? If so, what is the numerical value? 4. What is the mass of the base beak in the mass spectrum of 2,3-dimethylpentane? 5. What is the m/z of the peak of second highest mass in the spectrum? How can it be accounted for? What was lost as a fragment from the molecular ion to generate this species? 4 Mass Spectrometry CHEM 211-07 6. How can the peak at m/z=71 be accounted for? 7. How can the peak at m/z=57 be accounted for? 8. How can the peak at m/z=43 be accounted for? 9. In the boxes below, draw the structures for the substances with masses of 43 and 57 and 71. Mass of 43 Mass of 57 Mass of 71 2. Fragmentation Patterns in Mass Spectrometry b. Molecules with Hetero Atoms 1. Alkyl Halides Remember to look for the M+2 peaks Molecular ions from alkyl halides (and from alcohols) undergo fragmentation by cleavage of the bond (alpha) to the carbon-halogen bond in a process called alpha cleavage and which is represented in Figure 3. The process is more common in alkyl chlorides than in alkyl bromides. It is not seen in aryl halides. CHEM 211-07 Mass Spectrometry 5 cleavage H C CH3 CH3 C CH3 : Cl: CH3 .. CH3 H C+ : Cl : .. CH3 CH3 . C CH 3 CH3 H C : Cl + .. Figure 3: Alpha Cleavage of Alkyl Chlorides The cleavage produces a resonance stabilized cation (non-bonded electrons from chlorine are shared with electron deficient carbon) and a very stable alkyl free radical. Often this phenomenon occurs so quickly that there is no or little evidence of a molecular ion in the mass spectrum. To fully understand this phenomenon you need to recall the relative stabilities of carbocations (ChemActivity 11D) and alkyl free radicals (ChemActivity 11E). (Tertiary is more stable than secondary is more stable than primary is more stable than methyl). The sequence of stability is the same for alkyl carbocations and alkyl free radicals. QUESTIONS: A Figure 4 shows the mass spectrum of 3-chloro-3-methylpentane. * 6 Mass Spectrometry CHEM 211-07 Figure 4: Mass spectrum of 3-chloro-3-methylpentane 1. What is the molecular weight of C6H13Cl? Can you find the molecular ion in the mass spectrum of 3-chloro-3-methylpentane? What is its numerical value? 2. In the boxes below, draw the structures for the fragments responsible for the peaks at m/z=91 and 93? Mass of 91 Mass of 93 3. What is the fragment responsible for the peak at m/z=55? Does it contain chlorine? Explain your answer. Draw its structure. CHEM 211-07 Mass Spectrometry 7 Complete the Following Exercises for Homework: 1. Figure 5 shows several alkyl halides, which on ionizations produce molecular ions of the formula ([RCl]+.. Show how each can undergo alpha cleavage. Draw all products. If there is more than one opportunity for a substance to undergo alpha cleavage draw the products from each cleavage. CH 3 H CH 3 C C CH 3 CH 3 Cl H CH 3 C CH 3 CH 3 C CH 3 Cl CH 3 CH 3 C CH 2 CH 2 CH 3 Cl CH 3 CH 3 C CH 2 CH 2 CH3 Cl CH 3 H CH 3 C CHCH 2 CH 3 Cl CH 3 H Cl C CHCH 2 CH 3 CH 3 Figure 5: Alkyl Chlorides 8 Mass Spectrometry CHEM 211-07 2. Figure 6 shows the spectrum of bromobenzene, C6H5 Br QuickTime™ and a TIFF (LZW) decompressor are needed to see this picture. * Figure 6: Mass Spectrum of Bromobenzene 2a. What are the molecular weights for bromobenzene? 2b. Can you find the molecular ions in the mass spectrum of bromobenzene? 2c. Draw the structure of the fragment responsible for the peak at m/z=77? 3. Figure 7 shows the mass spectrum of bromocyclohexane. CHEM 211-07 Mass Spectrometry Figure 7. Mass Spectrum of Bromocyclohexane 3a. What is the molecular formula for bromocyclohexane? 3b. What are the molecular weights for bromocyclohexane? 3c. Can you find the molecular ions in the mass spectrum of bromocyclohexane? 9 10 Mass Spectrometry CHEM 211-07 3d. Draw the structure of the fragment responsible for the peak at m/z=83? CHEM 211-07 Mass Spectrometry 11 USING SPECTRABOOK We will use the SpectraBook program to look at the MS of a series of compounds in order to get a feel for how and hopefully why the radical cations initially produced in GC-MS analyses fragment the way they do. The intent is to use this latter information to become more fully informed on the ways by which electrons become more stable. 1. Examine the MS of 2-butanol (SpectraBook #1) and 3-pentanol (SpectraBook #2) and compare the fragmentation patterns. 1a. What is the molecular ion for each and is it visible? What are the numerical values for each? 1b. What is the base peak for each? 1c. Are they the same? 1d. Draw the structure of the species responsible for the base peak? 1e. Account for the differences and similarities in the two spectra. 2. Examine the MS of 3,3-dimethyl-2-butanone (SpectraBook #1) and 4heptanone (SpectraBook #1) and compare the fragmentation patterns. 2a. What is the molecular ion for each and is it visible? What are their numerical values? 2b. What is the base peak for each? 2c. Are they the same? 12 Mass Spectrometry CHEM 211-07 2d. Draw the structure of the species responsible for the base peak? 2e. Account for the differences and similarities in the two spectra. 3. Examine the MS of ethyl acetate (SpectraBook #2) and acetophenone (SpectraBook #2) 3a. What is the molecular ion for each and is it visible? What are their numerical values? 3b. What is the base peak for each? 3c. Are they the same? 3d. Draw the structure of the species responsible for the base peak? 3e. Account for the differences and similarities in the two spectra.