mass_spectrometry
advertisement

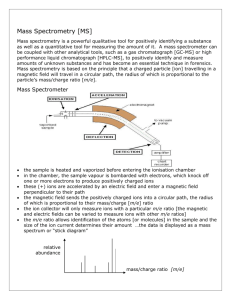
Unit 2 Chemistry Notes Mass Spectrometry page 1 of 6 Module 2 Mass Spectroscopy Diagram of a mass spectrometer Stage 1: Ionisation The sample is injected as a vapour and the atoms are bombarded by fast moving electrons. This causes the molecule to fragment into two or more pieces. When a molecule or part thereof fragments, one portion would be positively charged while the second portion will be uncharged. All uncharged fragments are ultimately lost in the machine as mass spectrometers only work with positive ions. Unit 2 Chemistry Notes Mass Spectrometry page 2 of 6 Stage 2: Acceleration The ions are accelerated so that they all have the same kinetic energy. Stage 3: Deflection Generally only univalent ions are present, so the only variable would be their masses and thus deflection is based on their mass on charge ratio (m/z) ratio. Stage 4: Detection The beam of ions passing through the machine is detected electrically. What the mass spectrometer output looks like The output from the chart recorder is usually simplified into a "stick diagram". This shows the relative current produced by ions of varying mass/charge ratio. The stick diagram for molybdenum looks like this: You may find diagrams in which the vertical axis is labelled as either "relative abundance" or "relative intensity". Whichever is used, it means the same thing. The vertical scale is related to the current received by the chart recorder - and so to the number of ions arriving at the detector: the greater the current, the more abundant the ion. NB relative abundance = percentage The origin of fragmentation patterns The formation of molecular ions When the vaporised organic sample passes into the ionisation chamber of a mass spectrometer, it is bombarded by a stream of electrons. These electrons have a high enough energy to knock an electron off an organic molecule to form a positive ion. This ion is called the molecular ion - or sometimes the parent ion. The molecular ion is often given the symbol M+ Unit 2 Chemistry Notes Mass Spectrometry page 3 of 6 Fragmentation The molecular ions are energetically unstable, and some of them will break up into smaller pieces. The simplest case is that a molecular ion breaks into two parts - one of which is another positive ion, and the other is an uncharged free radical. The uncharged free radical will NOT produce a line on the mass spectrum. Only charged particles will be accelerated, deflected and detected by the mass spectrometer. These uncharged particles will simply get lost in the machine eventually, they get removed by the vacuum pump. The ion, X+, will travel through the mass spectrometer just like any other positive ion - and will produce a line on the stick diagram. All sorts of fragmentations of the original molecular ion are possible - and that means that you will get a whole host of lines in the mass spectrum. For example, the mass spectrum of pentane looks like this: The molecular ion peak and the base peak In the stick diagram showing the mass spectrum of pentane, the line produced by the heaviest ion passing through the machine (at m/z = 72) is due to the molecular ion. Note: You have to be a bit careful about this, because in some cases, the molecular ion is so unstable that every single one of them splits up, and none gets through the machine to register in the mass spectrum. If there is no molecular ion peak, it means that the original molecule is highly branched. The tallest line in the stick diagram (in this case at m/z = 43) is called the base peak. This is usually given an arbitrary height/value of 100, and the height of everything else is measured relative to this. The base peak is the tallest peak because it represents the commonest fragment ion to be formed - either Unit 2 Chemistry Notes Mass Spectrometry page 4 of 6 because there are several ways in which it could be produced during fragmentation of the parent ion, or because it is a particularly stable ion. Checkpoint A Look at the mass spectrum of pentane above on the previous page. 1. Write the molecular formula of the fragment for m/z = 57. 2. What fragment would correspond to the m/z ratio of 72? What is the M+1 peak? If you have a complete mass spectrum, you will find a small line ONE m/z unit to the right of the molecular ion peak. This is called the M+1 peak. The M+1 peak is caused by the presence of ONE 13C isotope in the molecule. 13 C is a stable isotope of carbon. Carbon-13 makes up 1.11% of all carbon atoms. If you had a simple compound like methane, CH4, approximately 1 in every 100 of these molecules will contain carbon-13 rather than the more common carbon-12. That means that 1 in every 100 of the molecules will have a mass of 17 (13 + 4) rather than 16 (12 + 4). END OF MASS SPECTROSCOPY Unit 2 Chemistry Notes Mass Spectrometry Practice Questions page 5 of 6 Unit 2 Chemistry Notes Mass Spectrometry 2. page 6 of 6