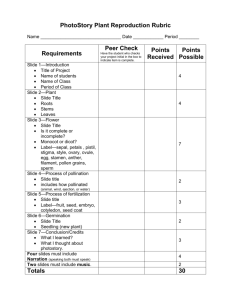
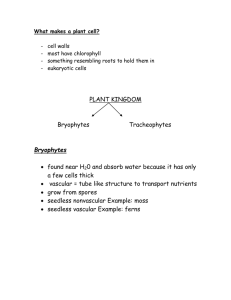
Genetic gain and gene diversity of seed orchard crops
advertisement

Introduction Seed orchards constitute an important component in most tree improvement programs, and seeds from seed orchards are superior to stand seeds. Nevertheless, it does not seem easy to achieve the optimal function of seed orchards because of many factors. Such factors are described, and general principles and practices of seed orchards are reviewed in this introduction. Overview of seed orchards 1. Seed orchard - general Reforestation can be achieved naturally or artificially. For artificial regeneration, seeds are collected from natural stands or from seed orchards. Seeds are important in both natural and managed populations as a resource of reproductive material and also for their commodity value in the production of forest products. To meet immediate seed needs, one can collect seeds from individually good phenotypes, good stands, seed production areas (seed stands) and proven seed sources (Zobel and Talbert, 1984). Long-term seed needs are often met by establishing seed orchards; greatly improved seeds are obtained from seed orchards. Seed orchards are the most common and cost-effective means of making available a stable supply of genetically improved seed (Varghese et al., 2000). A seed orchard is defined as an area where seeds are mass-produced to obtain the greatest genetic gain, as quickly and inexpensively as possible (Zobel et al., 1958). Also, it is defined as a plantation of selected clones or progenies which is isolated or managed to avoid or reduce pollination from outside sources, and managed to produce frequent, abundant, and easily harvested crops of seed (OECD, 1974; Feilberg and Soegaard, 1975). In study VII, seed orchards are defined as the production populations where genetic gain from tree breeding is transferred into planting materials and to commercial forest crops. The objectives of a seed orchard and methodology for its establishment may be modified when seeds are not needed for immediate use, but where there is a perceived future need for seeds. Clonal propagation techniques have been improving in effectiveness, both biologically and economically, for many years. These may influence the nature of future output systems and modify the present role of seed orchards (see review by Sweet, 1995). In the future, mass propagation techniques, like tissue culture, somatic embryogenesis and artificial seeds, may make it possible to get seeds or plants directly from in vitro conditions. In the foreseeable future, however, the role of seed orchards as a primary production system for genetic improvement programs is likely to continue. 8 2. Seed orchard establishment and management Seed orchards may be established vegetatively with grafts, cuttings, rooted cuttings or tissue culture plantlets propagated from a selected tree, or with seedlings produced from seeds of the selected parent (El-Kassaby and Askew, 1998). In either case, a careful testing and evaluation program can be used to separate the environmental and genetic factors associated with the trees’ superiority. Seedling seed orchards (SSO) have a broad genetic base because of the large number of parents involved, but the selection differential is less than in the vegetative seed orchard. When the seedlings are grown from open-pollinated seeds collected from selected trees, the cost for raising material and time required for establishment will usually be low in comparison with costs of establishing clonal seed orchards (Toda, 1964). Seedling seed orchards are suitable for species like most Eucalyptus spp., Picea mariana, the early flowering pines, and many hardwoods that produce seed at a young age. In seedling seed orchards, some progenies may outgrow others that may increase inbreeding. The breeding seedling orchard (BSO), a derivative of the SSO, is a flexible strategy that lies somewhere between a SSO and a progeny test (Barnes, 1995). In the BSO, the conventional hierarchy of sequential testing, selection and seed production populations is combined in a single planting. This may be a cheaper and less complicated way to run a tree improvement program. It can provide the potential to respond to new materials and new demands, and allows the breeder freedom to be adventurous without taking unacceptable risks. In clonal seed orchards (CSO), there may be some level of graft incompatibility, but the problem has simply been accepted. On the other hand, grafting is particularly useful for species where flowering is delayed, as grafts retain the physiological age of the parents. Root deformities and low productive output may also be problems in seed orchards established with cuttings or by tissue culture. The possibility of selfing is considerably greater in vegetative orchards, but this can be minimized by keeping ramets of the same clone well separated. The decision whether to use clonal, seedling or polycross seed orchards should be made on the basis of economic and genetic considerations (Sweet, 1995). The choice of seedling versus clonal seed orchards depends primarily on the earliness of flowering of grafted trees and of seedlings, the relative difficulty, cost and speed of establishing grafted trees and seedlings, and selection differential possible with clones and families. If trees are moderately self-sterile and have overlapping flowering periods (e.g., in Acacia and Populus species), it should be possible to produce significant quantities of hybrid seeds in seed orchards (Griffin et al., 1992). Some trees reveal high specific combining ability (SCA) in certain combinations. If they are 9 outstanding for certain characteristics, such as volume production, tree form and disease resistance, they can be used for the establishment of bi-clonal seed orchards. There is considerable interest in taking advantage of these combinations by establishing two-clone seed orchards, the progeny from which will be planted locally or on specific problem areas (Kellison, 1971; Sedgley and Griffin, 1989). Determination of the number of families or clones to be included in a seed orchard depends on the short- and long-term goals of the breeding program (ElKassaby and Askew, 1998). Breeding programs that use seed orchard genotypes as parents for the next successive generation will reduce the effective population size just a few generations if a small number of parents is used. Also, the gene diversity may soon become low (VIII; Bila, 2000). If breeding (e.g., clonal archives) and production populations (e.g., seed orchards) are maintained separately, the breeding population must reflect the diversity of the original population and be sufficiently large to maintain gene diversity for many generations ahead, whereas the production population can be managed to meet specific needs, e.g., high gain can be combined with an acceptable gene diversity in a production forest. To guarantee pollination within an orchard and the success of seed orchard material in reforestation, seed orchards should be established with superior genotypes originating from geographical distribution areas (Sarvas, 1970, Koski 1980b). In other words, the location of a seed orchard should be geographically within the natural range of the species, but transfer outside this area to a warmer climate in the south can be advantageous for seed maturation, earlier flowering and physical isolation from pollen contamination (Sarvas, 1970). Moreover, transfer from high to lower elevations should be used with similar effects in cases where there are appreciable differences in altitude (Hellebergshaugem, 1970). Moving orchards should be done only after consideration that it seems likely normal fruit and seed are produced in the new environment. It has also been observed that maternal environment might influence growth and growth rhythm of the offspring, the so-called “after-effect” (Bjornstad, 1981; Dormling and Johnsen, 1992; Lindgren and Wei, 1994). This may be a factor that deserves consideration in seed orchard localization. Frost, drought and wind may all have adverse effects on the establishment of orchards, and/or flower crops and seed set. Of key importance is whether the environment of an area favors the production of seed crops. An orchard site should be intermediate in fertility. Fertile sites often involve problems with heavy vegetative and poor reproductive growth. Sloping ground creates problems with management and cone harvest. Abandoned agricultural field, flat terrain and gently sloping land can thus be good (Zobel and Talbert, 1984). Damage by animals is also a problem, and could be considered in choosing the spot where to 10 place a seed orchard. Cold air pockets may be a problem, especially where the land looks flat that deserves attention. Seed orchards must be protected or isolated from contamination by outside pollen. Pollen dilution zones are most critical for advanced-generation seed orchards because of the greater potential loss of genetic gain. Conversely, contamination from foreign can increase seed set as a young orchard first comes into production, when pollen production is typically low, and can increase genetic diversity of seed crops. Concerning protection, it is good to orient the long axis of the orchard with the direction of the prevailing wind at the time of pollen dispersal if a rectangular configuration is used. Most of the pollen from a single tree is dispersed within a very short distance. Even so, considerable amounts may migrate from several hundred or thousand meters away during a year of very heavy flowering (Silen, 1962). For most transects, there is a very rapid decrease in pollen density within 400-600m from the edge of the pollen source, beyond which the pollen frequency is fairly constant (Andersson, 1955). However, efforts to isolate seed orchards have not been very successful and pollen contamination is often large (Lindgren, 1991). Collecting seed crops is an expensive process. To facilitate seed collection, the crowns of orchard trees should be pruned to encourage the development of a short, wide and bushy habit except for those species where fallen seeds are collected from the ground, such as oak and beech. Pruning must be done with care, as it can reduce cone production or increase male flowering. The loss of seed production to insects, disease, mammals and birds should also be minimized. How do we determine when cones are ripe and ready for harvest? Key to insuring efficient cone harvest is recognition of cone maturity. Harvesting mature cones will do much to promote proper cone opening and extraction of high quality seed. If cones are harvested while immature, they will fail to open properly, seed yield per cone will be reduced, and seed viability will be diminished. Sometimes, a remedy may be found in the proper treatment of the cones after cone collection, so the seeds can mature after collection. As seeds of conifers become mature, the specific gravity of the cones decreases because of water loss. This change in specific gravity provides one of the most reliable tests for determining ripeness of conifer cones (Hartmann et al., 1997). It has been found that seeds from cones harvested 3 weeks prior to cone maturity are physiological mature in a loblolly pine, but premature harvesting of the cones results in case-hardening (Matthews, 1963). Cells on the top and bottom of the cone scales dry at different rates, causing the scale to flex outward thereby releasing the seed. In some cases, accidental mislabeling of grafts or missing identification of rootstocks that developed into trees may cause variation in the number of ramets among clones (e.g., Kossuth et al., 1988; Paule, 1991) and in the estimates of genetic composition of seed orchard crop (VII). Even if these errors become 11 apparent, the mislabeled materials must be identified or discarded. Harvesting seeds from alien genotypes and even leaving alien ones in the seed orchard leads to a substantial contamination of the gene pool of the orchard crop (Gömöry et al., 2000). This may increase gene diversity, but the effect would be marginal compared to the loss in genetic gain. For establishment clonal seed orchards, there are many possibilities for errors, starting from collecting and labeling the scions, over the manipulation during the transport, grafting, handling the material in the nursery, up to planting (Gömöry et al., 2000). Scions may also fail, leaving only the rootstock to develop a seedproducing crown. These mislabeled or alien ramets represent genotypes of unknown genetic quality. So, the presence of any alien material must be considered as in internal contamination of the orchard gene pool. A rapid and non-destructive means of clone certification is needed, using biochemical markers such as monoterpenes or isozymes that are not realistic in practical operations (Kossuth et al., 1988). DNA fingerprinting for confirmation of clone identity may be readily available soon, but it may not be cheap. 3. Seed production and quality in seed orchards The progress of a seed orchard program depends on a plentiful delivery of viable seed. The final cone and seed yields may be influenced by breakdown of any one of the processes of pollination, pollen grain germination, pollen tube growth, fertilization and embryo development (Brown, 1971). Seed yield is also reduced if insufficient pollen is supplied to the female strobilus. Unpollinated ovules degenerate during the first growing season, and only the wings continue to develop in most conifers (Sarvas, 1962). For maximum yield of seed, the female strobili must be pollinated when they are fully receptive to pollen, i.e., when the axis of the strobilus is fully elongated and the ovuliferous scales are separated from each other. Empty seed and seed of low viability may also result from a breakdown in embryogeny. A further loss of seeds is occasioned by premature abscission of the cones from the tree. There has long been interest in the flowering behavior of forest trees. The development of seed orchards for production of improved seeds has intensified this interest and focused attention on individual clones. There are many desires for a well functioning seed orchard and still more to get it to work as an ideal object (II, V, XI; Sarvas, 1970; Eriksson et al., 1973; Jonsson et al., 1976; Koski, 1980b; El-Kassaby and Reynolds, 1990; Wheeler and Jech, 1992; Harju, 1995). Most existing orchards have been established based on the assumption that each clone and ramet (or family-plot and seedling tree) in the orchard would 12 flower during the same period (synchronization, random mating), have the same cycle of periodic heavy flower production, be completely inter-fertile with all its neighbors and yield identical numbers of viable seed per tree, have the same degree of resistance to self-incompatibility, and have a similar rate of growth and crown shape as all other trees. Non- or minimum relatedness and pollen contamination are also expected. It is almost certain that these assumptions are virtually never satisfied. On the other hands, there are not likely limited deviations from these assumptions, which lead to serious consequences. But it is difficult to formulate the consequences numerically. Thus, it is important to evaluate their impact and consider them properly when establishing and managing seed orchards. The biological impact of forest tree reproductive processes and the extent of management practice of seed orchards and nurseries on the genetic representation of new forests were reviewed by Edwards and El-Kassaby (1996). The first problem related to seed production for seed orchard programs is to estimate the amount of seed needed in the future. A buffering amount should be calculated for loss or failure of seed production because good seed crops will not be obtained every year. Also, the time in seed storage (at least 2-3 years) should be considered if storage is possible. Zobel and Talbert (1984) suggested that 30% greater amount than currently predicted requirement would be for a sufficient buffer in planning the target amount of seed from seed orchard programs. The buffering may also be obtained by keeping older seed orchards for a longer time or creating options to produce more seeds in cheaper ways if needed. Trees may grow more quickly (fast DBH growth) and produce more cones under CO2 enrichment because developing seeds are strong carbon sinks (Lawlor, 1991). It is recently reported by LaDeau and Clark (2001) that trees are twice as likely to be reproductively mature and produce three times as many cones and seeds as trees at ambient CO2 concentration, as a result of 3 years’ CO2 fumigation in a Pinus taeda half-sib plantation. This implies that trees in seed orchards can produce plenty of seeds at early ages and smaller sizes if the application of this technique is sustainable and cost-effective. Seed orchards have been established with selected superior trees to produce seeds that are physiologically and genetically better than those obtainable from natural stands (Harju, 1995). The degree of superiority is in general determined by comparing the performance of the progeny of the orchard trees with the performance of a checklot family in a carefully designed test (Li et al., 1999). Due to the deviation from the ideal situations, the improvement of seed quality cannot be accurately calculated and does not reach at the maximum. The minimum requirements can thus be set and allowances made for deviations from the ideal situations (Koski, 1980b). 13 Forest trees are generally still in early stages of domestication. Most tree breeding programs were initiated in the late 50’s or 60’s. The first-generation seed orchards were established using ramets or seedlings of selected plus-trees without further genetic information (Hodge and White, 1993). Usually, the intention was to use as an equal number of ramets, but there were often deviations for practical difficulties (VII). Improved seed orchards (1.5 or 2.5 generation SO) consist of the very best genotypes, based on testing of their progeny. No matter what kind of seed orchard is established, clone or family records must be kept to ensure use of the best genotypes and to minimize deleterious related matings, especially in the advanced-generation seed orchards where genetic information from progeny tests is available and parents will be more closely related and perhaps inbred. Orchard managers want seed orchards with well-pruned trees as in apple orchards, using topping and branch pruning, but trees easily grow beyond reach for convenient cone collection. Several methods of cone collection are currently practiced in seed orchards; they include the use of ladders, mechanical lifts, netting, vacuum machines, tree shakers and helicopters (Ho and Schooley, 1995). Well-trained small animals (e.g., monkeys) can also be used. Among them, ladders and ground netting are generally considered as the cheapest and most practical methods. Tree climbers may be employed, but this method is expensive, and the branch apices that bear cones are often difficult to reach safely. Thus, it seems increasingly more unrealistic to rely on human climbers. The use of special stocks, often of a different species or variety from the scion, has been practiced in fruit and forest orchards to reduce the stature of the grafts and to encourage early fruiting and flowering (Gardner et al., 1922). The ability to produce broomy, dwarf trees may be of substantial value in grafted seed orchards (Buckland and Kuijt, 1957). The development of dwarfing rootstocks is therefore an important concern in seed orchard technology, Christmas tree culture and horticulture (Duffield and Wheat, 1963). Use of root suckers as stocks may also be of benefit to reduce the height of seed trees. Some grafted scions do not develop satisfactorily due to some reasons such as failure in graft development and inadequate vascular transport of water and nutrient through xylem tissues (Cameron and Thomson, 1971), so-called graft incompatibility or uncongeniality (Slee and Spidy, 1970). In less severe cases, incompatible grafts cannot be detected for some time after establishment, and their occurrence modifies irrevocably an orchard layout, disturbs clone representation (VII) and reduces seed production. Also, there is a large variation in incompatibility among clones and among species (Ahlgren, 1972). Dyson (1975) reported that a large proportion of the ramets on Pinus radiata stocks had a dwarf, bushy form caused by graft incompatibility, but appeared to be bearing more ripening cones than grafts made on Pinus patula stocks in P. patula clonal seed orchards. Heteroplastic grafts in a black pine (P. nigra) seed orchard 14 displayed more and better seeds than homoplastic ones (Climent et al., 1997). Therefore, if grafts are made with genetically similar materials, the problems associated with graft incompatibility may not be serious in clonal seed orchards. Seeds are often transferred between countries or between areas within countries. For the trade or transfer, adequate information about source and history should accompany the shipment (Jones and Burley, 1973; OECD, 1974). Certification is defined as the authoritative attestation of facts or statements and it usually implies documentation by a formal written certificate. Seed certification is an official statement that a seed lot conforms to certain standards, which may include specific identity, origin, genetic characters and seed purity (Muhs, 1986; Portlock, 1988). In seed orchards, seed certification implies, in general, a uniform, high genetic quality that may be described by genetic gain, gene diversity and germination ability. An effective number, which gives better genetic information, is recommended to describe gene diversity of orchard populations and orchard crops (VII). Genetic gain Genetic improvement includes selection, testing and breeding from the species, through the population and family, to the clonal level (see Fig. 3; Barnes, 1995). The amount of improvement that can be made by plus-tree selection among a number of initial parents depends on the amount of genetic variation among the parents. In general, there is great variation found within and among populations in natural forests. By selecting plus-trees and using them to establish seed orchards, one can get genetic gain in seed crops. In the orchards, the gain made by the plus-tree selection is realized from the general combining ability (GCA) of the selected trees, which comes from the additive variance in the reference population. For gain from specific combining ability, parents are chosen for crossing and the best combinations are selected (cf. a bi-clonal seed orchard). But, the specific combining ability is a reflection of dominance and epistatic gene actions. So, its value cannot be predicted from phenotypes before the cross is made (Zobel and Talbert, 1984). To get high intensity of selection and to maintain high diversity require a large number of parents, therefore more parents than needed are used in the first-generation seed orchards (Hodge and White, 1993). This also facilitates genetic thinning on later stages. In advanced-generation orchards, breeding values and/or fertility variation of orchard parents will be known from genetic testing or fertility assessment. With knowledge of breeding values and other genetic information, it would be possible to establish new seed orchards using intentionally different numbers of ramets per clone or progenies per family to attain the optimal genetic gain with desirable diversity (VII; ‘linear deployment’ by Lindgren and Matheson, 1986; ‘step-wise’ 15 Selected plus trees Selection from natural stands or unimproved plantations Genetic gain by selection (7% in volume) Progeny population Progeny test Crossing between superior genotypes 1st generation seed orchard Genetic information Genetic gain by roguing inferior genotypes or by collection best ones (12%) Information Additional gain by repeated roguing Improved SO (1.5 gen.) Selection and crossing; Genetic gain Information Genetic gain nd 2 generation seed orchard Infusion (new genetic materials) (13-21%.) Genetic gain by roguing to keep the best 30% (26-35%) Improved SO (2.5 gen.) Figure 1. Flow chart of the generation of seed orchards. Values for genetic gain are from Li et al. (1999). 16 relationship by Hodge and White, 1993). These optimal and step-wise solutions could result in more gain than truncation selection, while minimizing the disadvantage connected to reduction in diversity. Establishing improved seed orchards by genetic thinning with high intensity may be a more effective way to get high genetic gain rather than establishing of new seed orchards, but some loss of gene diversity is unavoidable after roguing (I). Li et al. (1999) reported that loblolly pine in the southern United States grown from seeds of first-generation seed orchards produced 7-12% more volume per unit area at harvest than trees grown from seeds of wild forests (Fig. 1), and that of second-generation seed orchards showed 13-21% gain in rotation volume. The estimated gain from rogued second-generation seed orchards varied from 2635%, depending on the breeding region considered. The genetic gain estimated by Li et al. (1999) could be over estimated because of the unequal contribution and relatedness among parents. In the Western Gulf Forest Tree Improvement program, roguing improved the performance of loblolly pine seed orchards by an average of 2.9% in breeding value for volume growth (Byram et al., 1998). On the other hand, Matheson et al. (1986) showed that Pinus radiata trees from first-generation seed orchards had considerably better growth (15-30%) than control seed lots. Sluder (1994) also reported that high gain in rust resistance and height growth could be obtained in a secondgeneration slash pine seedling seed orchard. Genetic gain can be related to fertility of orchard parents (Griffin, 1982). In practice, orchards are culled on the basis of breeding value of the parents, rather than their fertility, but one should predict to what extent is reduction of total seed production likely as a result of clonal selection on the basis of breeding value. It may thus be necessary to give some weight to the fertility or collection cost as well. If general combining ability (GCA) and fertility are known, the expected genetic gain can be expressed as G GCAi pi N i 1 [1] where N is the census number and pi is the fertility of ith parent in the seed orchard. If parents contribute equally to the seed orchard crop, genetic value and gene diversity in bulked orchard seeds are expected to be the same as that of the orchard parents. Due to fluctuations in fertility, however, genetic gain and gene diversity are difficult to predict accurately (Griffin, 1982). Gene diversity and group coancestry The accumulated loss of gene diversity is the group coancestry (Lindgren and Kang, 1997). The gene diversity can be estimated relative to the reference 17 population (II and VII). The reference population is defined as having an infinite number of unrelated individuals, and thus a group coancestry of 0 and gene diversity of 1. No genes of individuals in the reference population are identical by descent. The inbreeding in the reference population is also considered to be 0 (III; Yeh, 2000). Loss of gene diversity may occur in two stages: (1) when seed orchards are established with a limited number of parents; and (2) when there is unequal contribution of gametes by the orchard parents (Harju, 1995). On the other hand, pollen contamination increases the gene diversity of orchard crop (Lindgren and Mullin, 1998). The term group coancestry was introduced by Cockerham (1967), who defined the group coancestry coefficient as referring to the probability of identity of a random pair of genes sampled, with replacement, among the 2N genes of the N individuals in a population. By definition, therefore, group coancestry is the average coancestry of all pairs of population members including individuals with themselves (self-coancestry). Group coancestry is equivalent to “average coancestry” or “average kinship”, as used in some studies (e.g., Askew and Burrows, 1983; Fries, 1994; Lacy, 1995), but other studies (or even in the same studies) also use the terms “average coancestry” or “mean kinship” referring to averages of other sets of values. As the term is used with different meanings by various investigators, without being intuitively clear as to which values are to be averaged, the term easily causes misunderstandings. Lindgren et al. (1996) coined “status number” calculated from the group coancestry, which gives clear intuition on the concept of effective number. Objectives The main aim of this thesis is to give a theoretical foundation for genetic aspects of seed orchards and their management. Several factors, such as selection intensity, genetic value, group coancestry, relatedness, fertility, selfing and gene migration, are considered in estimating and predicting genetic quality of seed orchard crops. Most emphases are placed on evaluating effects of fertility variation. Much of the theory heads at evaluating methods for efficient orchard management. The following questions are addressed: 1. How can seed orchard managers predict and improve the genetic quality of seed orchard crops and modify the balance between genetic gain and gene diversity? (I) 2. If parents do not contribute equally, what are the effects and consequences of unequal contribution in transferring genes to the planted forest? Is it possible 18 to predict genetic composition of the seed crop by the assessment of female and male strobilus production? (II, III and V) 3. What are the implications of fertility variation in the management of seed orchards? If the parental fertility is different between genders, what is the consequence of correlation on the gene diversity of orchard crops and on the management of seed orchards? (III and VI) 4. How large is the fertility variation in the real populations of forest trees or clones, and what kinds of factors can have importance in the real populations? (IV) 5. Why do fertility variation and ramet variation occur? (VII) and what are the consequences of relatedness, selfing, parental number and pollen contamination in transmitting genes from orchard parents to their offspring? (VIII and IX) 6. How can breeders or conservationists decide the optimum tradeoff between gene diversity (status number) and seed production by balancing and/or controlling maternal fertility in seed collections? (X) 7. When there is relatedness and fertility variation among orchard parents, how do these variables affect the gene diversity of seed crops? What is the effect of gene migration from extraneous pollen sources? (IX and XI) 19 Materials and methods The main purpose of this thesis was to develop breeding theory in seed orchards. New theoretical methods were developed and documented in the included publications. The methods and models were applied for various cases, using empirical data and information on flowering and ramets from different species, years and countries. Flowering and ramet data Numbers of female and male strobili were counted in Pinus densiflora and Pinus thunbergii clonal seed orchards that are located on Anmyon island (36o30’N, 126o20’E, elevation 35 m), which is the western part of Korea. Flowering data from the clonal archive of Pinus koraiensis were also collected for consecutive five years from 1991 through 1995, which was established in Suwon (37o70’N, 127o20’E, elevation 100 m). All grafts originated from plus trees selected throughout Korea. Strobilus production was assessed by counting all female strobili, and by estimating the number of male strobili. Flowering assessment was then used to estimate fertility and fertility variation (II, III, V, VI and XI). Data collection has been done almost every year in Korean seed orchards. Four-year observations were used in paper V, and fiveand six-year data in papers XI and VI, respectively. Fertility variation among species was estimated and compared using a single year observation from three seed orchards in different species in study II. The term ‘fertility’ is defined as the capacity of an organism to produce living offspring (Krebs, 1985). The notion of fertility is the actual level of performance in the population based on the number of successful progeny, which is a measure of physiological or genetic capacity. The similar term ‘fecundity’ is the potential level of performance of the population, or physical capacity. In the present study, the female and male fertilities are measured as the number of female and male gametes produced by an individual relative to others in the orchard population (Gregorius, 1989). In study VII, we used orchard records from a large number of seed orchards in Finland, Korea and Sweden, in order to estimate ramet variation and to predict its effect on the gene diversity of orchard crops. All registered seed orchards of Pinus koraiensis and Pinus densiflora in Korea, and Pinus sylvestris and Picea abies in Finland were used to study variation in ramet numbers among parents. Not all, but many Swedish seed orchards were also included. Unlike in Korea and Finland, orchard records in Sweden are not kept centrally and orchards may have several owners. Newer seed orchards must be registered at the Swedish National Board of Forestry and this documentation includes ramet numbers. Many records 20 were available, either directly from the orchard owners, or via the Swedish Forest Research Institute (SkogForsk). Theoretical methods 1. Relatedness among seeds from seed orchards Pedigree and relatedness among orchard crops from a seed orchard are illustrated in Figure 2. Coancestry is a quantitative description of relatedness. AB means the coancestry between individuals A and B, and it is 0 between unrelated individuals (Fig. 2-b). In the case of self-coancestry, i.e., an individual’s coancestry with itself, AA= 0.5(1+FA), where FA is the coefficient of inbreeding for the parent A. When the parent is not inbred, the value of FA is zero. Self-coancestry can also be understood as the group coancestry for a population with a single member (Fig. 2-a), thus an extrapolation of the ordinary coancestry concept. So, the group coancestry of a non-inbred individual (FA= 0) is 0.5. A a) self-coancestry (A,A)= 0.5(1+FA) (Y1,Y2)=0.25(AA+2AB+BB) A A B C D Y K b) different parents A B X1 X2 X1 Y d) between self-sibs e) self and outcross sibs (X1,X2)= (A,A)= 0.5(1+FA) (Y,Z)=0.25(AA+AC+AB+BC) A B A Y1 Y2 c) full-sibs (Y,K)=0.25(AC+AD+BC+BD) B A C Y Z f) half-sibs (X1,Y)=0.5(AA+AB) Figure 2. Pedigree and relatedness (coancestry, ) among orchard seeds: a) selfcoancestry; b) unrelated seeds; c) full-sibs; d) self-sibs; e) double pairing of a parent; and f) half-sibs. FA represents the coefficient of inbreeding for parent A. In figure 2-d), it looks like self-sibs. If the seed parent A were not inbred, relatedness between selfed seeds would be 0.5. It will be higher when the parent is inbred. In some papers, it was assumed that the seed parents were unrelated and non-inbred (II, III and VI), but this was not so in papers IX and XI. 21 Under the idealized situation of non-relatedness and equal contribution among parents, the gene diversity of orchard crop will be maximized. Gene diversity is reduced if there is relatedness or unequal fertility among orchard parents due to the occurrence of sib relationships (IX and XI). This can be compensated for by having more parents in the seed orchard, but then the gain may be reduced (III). 2. Fertility variation estimation Each orchard genotype has a fertility value (pi), the ability to produce successful gametes. Fertility can be regarded as the progeny mothered or fathered by an individual relative to others in the population (Devlin and Ellstrand, 1990). The “sibling coefficient” () is defined here and estimated directly from observations as the sum of squared parental contributions multiplied by the census number (see also Table 1). Expected fertility of an individual is an average of maternal and paternal fertilities as follows (II and III): f mi Ψ N p N i i 1 i 1 2 N 2 i N 2 2 where N is the census number, fi is the fertility as female and mi is the fertility as male in a genotype i. The sibling coefficient () was denoted as A in papers I, II, III and VII. Note that pi2 is the probability that two genes in the offspring come from the same parent i, summed over all parents, i.e., the likelihood that two gametes in the gamete pool originate from the same individual. The sibling coefficient expresses how fertility varies among parents as the increase in the probability that sibs occur compared to the situation where parents have equal fertility (III). The sibling coefficient cannot be smaller than one. If = 1, all individuals have the same fertility. If = 2, for instance, it means that the probability that two individuals share a parent is twice as high, compared to when the parental fertility is equal across the population. Fertility differences among population members can also be described by the coefficient of variation (CV) in fertility, so that CV 2 ( N 1) Ψ 1 N 3 where N is the size of sample (II and IV). When fertility is measured from all population members (III and IX), then N disappears from formula [3] and the sibling coefficient will hence be = CV2 + 1. The sibling coefficient carries the same information as the coefficient of variation, but is based on a probabilistic 22 aspect, while CV is based on a variance aspect. Also, relates to the orchard crop or offspring, while CV refers to the parental population. Therefore, may have the potential to give genetic information for describing gene diversity of orchard crops that is closer to the seed crop than is the CV. Fertility variation occurs, not only among seed parents, but also among pollen parents. Thus, the sibling coefficient for parental fertility can be divided into female (f) and male (m) components as N f N f i 2 CV f2 1 and i 1 [4] N m N mi2 CVm2 1 i 1 where CVf and CVm are the coefficients of variation in female and male fertilities, respectively. Fertility can also be described by a probability density function f(x), where x is the percentile of trees ranked by fertility and f(x)dx= 1. For a large population of trees, p= f(x)/N is the predicted fertility of the parent with rank corresponding to x (II). An example of a density function is the power function, F(x)= xa for a≥1. The derivative of the power function is f(x)= axa-1. For a given population, an individual fertility (pi) can also be estimated in a relative sense, based on the sum of observations. Parents are then ranked for the fertility and transformed to cumulative contributions summing up to one. Cumulative contributions are plotted against the parents’ proportions, and then the a value that gives the bestfitting curve is chosen (Bila, 2000). The exponent of the fitted power function, a can also be expressed as a function of (II and X), thus there may not be direct need of the parameter (a) fitted to empirical data because is enough to specify a well-fitted power function. is related to the exponent a as 1 a Ψ 1 1 Ψ [5] 3. Group coancestry and status number Status number (Ns) is defined as half the inverse of group coancestry () (Lindgren et al., 1996). 23 Ns 0.5 [6] An attractive property of status number is that it is equivalent to the census number for a population of unrelated, non-inbred individuals. The status number describes an equivalent population of unrelated, non-inbred individuals, where the probability to draw two genes identical by descent is the same as for the population under study. It is thus regarded as an effective number (II). Status number calculations were based, not only on relatedness among parents and pollen contamination from extraneous undesirable sources (IX and XI), but also on ramet variation (VII) and fertility variation (II and III). Relative status number (Nr), which is the relationship between Ns and N, was calculated as Nr = Ns/N = 1/ (II, III, VII and X). The relative status number relates to how the seed orchard is functioning in terms of relatedness or fertility variation; thus, it is a convenient way of describing the orchard situation from the management point of view. When the relative status number is low, orchard managers should consider some action to improve the efficiency of seed orchard. The effective number of parents (Np), as a function of fertility variation ( ), was calculated (III and VI) as Np N Ψ [7] By knowing the magnitude of fertility variation among genotypes that will consist of a seed orchard, the census number can be chosen to achieve satisfactory gene diversity (IV). The effective number of clones, Nc (c.f., status number) was also calculated, based on the variation of ramet numbers among clones that are unrelated and noninbred (VII), as Nc ntotal N ni i 1 1 1 N N n ri total i 1 i 1 ni [8] where ntotal is the total number ramets in the seed orchard, and ni is the number of ramets of clone i. When relative values (ri) are used, the effective number of clones becomes the status number of orchard crops where parents are unrelated, non-inbred and parental fertility is proportional to the ramet number (VII; Lindgren and Mullin, 1998; Nikkanen and Ruotsalainen, 2000). 24 4. Symbols and definitions The following list of symbols is used throughout the thesis (Table 1). More complete definitions and rules of operation for seed orchard populations are given in the individual chapters. Table 1. List of symbols, terms and definitions of main parameters used in the study Symbol Term Definition G genetic gain i selection intensity M gene migration Rate of immigrant genes from outside the seed orchard. It is half the pollen contamination N census number Total number of genotypes (in a seed orchard) Ns status number Half the group coancestry (=0.5/; see Table 5) Nr relative status number Ns / N; proportion of the status number to the census number f inbreeding coefficient F (group) inbreeding coefficient coancestry Probability of alleles being identical by descent in an individual; the chance that both homologous genes in the same zygote are identical by descent Average inbreeding coefficient among individuals in a population Change in the average breeding value (additive genetic value) achieved by artificial selection Number of standard deviations by which the mean of the selected individuals exceeds the mean of the base population Probability that genes, taken at random from each of the concerned individuals, are identical by descent (= coefficient of kinship). group coancestry Average of all possible coancestries between individuals in a group, including self-coancestry sibling coefficient Probability of two random alleles being identical by descent in a set of gametes from the same group. It is designated in some papers as A. 25 t 26 generation Time, measured in generations. Initial members of the source population (initial generation; t= 0) are always assumed to be unrelated and non-inbred. Summarized results and discussion All publications included in this thesis have their unique results and consequences on genetic gain and gene diversity of orchard crops, and on the management of seed orchards. In this chapter, therefore, results from these papers are summarized and discussed from various genetic and management viewpoints. Genetic gain Genetic gain is always connected to gene diversity, as artificial selection is to genetic diversity. There are both short-term and long-term considerations in practical tree breeding programs (Fig. 3). In the short term, forestry practices should result in productive stands that are able to tolerate changing environmental pressures for the duration of the rotation. The long-term concern is the maintenance of reservoirs for genetic variability, which is needed for current breeding populations, but still more important to meet future needs (Savolainen and Kärkkäinen, 1992). In short-term breeding, genetic gain is usually maximized in clonal forestry, family forestry and seed orchards. For long-term breeding strategy, breeding and base populations should be managed for sustainable genetic diversity. Higher genetic gain CF Short-term SO Breeding pop. Long-term Base population population Base Broader genetic diversity Figure 3. Relationship between genetic gain and diversity on the pyramid of improvement in any tree-breeding program. CF represents clonal forestry with a few superior clones and SO represents seed orchards. Genetic improvement can be defined as a process whereby genetic value is improved while joint consideration is given to the genetic diversity of deployed material. As shown in Fig. 1, genetic gain in seed orchards can be achieved at different stages from plus-tree selection through roguing inferior parents and/or crossing superior parents. Plus-tree selection itself gives some extra gain (maybe 27 1-2%) compared to natural stands, due to the avoidance of relatedness as the plus trees are usually selected in different stands. In seed orchard management, there are different techniques that may be used to increase genetic gain while retaining gene diversity, including selective cone harvest, genetic thinning and/or a combination of these techniques. In study I, genetic gain and gene diversity of orchard crops from clonal seed orchards were formulated considering selection intensity, genetic value, fertility variation, pollen contamination, and inferiority of the alien pollen. Formulae were derived to calculate genetic gain (also gene diversity) and applied to five management strategies. The formulae and results of the study I can be used for identifying favorable alternatives in the management of seed orchards. The main results and discussion follow. By truncation selection, the expected breeding value of the orchard crop (G) after selection can be predicted as G ie A MC 0.5 i( N f , N ) (1 2M )i( Nm , N ) A MC [9] where ie is the effective selection intensity, the average selection intensity applied to female and male parents; A is the standard deviation of breeding values for orchard clones; M is the gene migration, that is half the pollen contamination; C is the contamination inferiority; and Nf and Nm represent the selected number of female and male parents, respectively. If selection for general combining ability is to be effective, clone or family differences must exist or, synonymously, the additive variance (A2) must be greater than zero (Namkoong et al., 1966). The predicted genetic gain is not affected by fertility variation if the breeding value is not correlated with fertility. While Schimidtling (1981) reported that a negative relationship between growth and flowering had a genetic basis in loblolly pine, Byram et al. (1986) found that there was no significant correlation between volume growth and cone production in loblolly pine seed orchards. A positive correlation was observed between vegetative and flowering characteristics in tamarind (Varghese et al., 2000). It seems that there is a trend of positive correlation between crown size and flower production, and negative correlation between tree growth and cone production (Nikkanen and Ruotsalainen, 2000). Plus-tree selection is mass selection; so seed orchard establishment itself gives genetic gain. Here, the scale for breeding value is defined so that the average breeding value of plus trees (thus initial seed orchard parents) is set to zero (I). Therefore, the genetic gain for orchard crops from the initial seed orchard will only be affected by gene migration (i.e., pollen contamination and its inferiority), 28 G = M*C. The influence of pollen contamination on the genetic value of the crop is usually negative, thus the contaminating pollen has a negative breeding value. 1. Selective harvest Seeds can be collected selectively, based generally on genetic values of the seed parents. In the case of open-pollinated orchards, no selection can be applied to the pollen parent. Genetic gain from the selective harvest will be G 0.5i( N m , N ) A MC [10] Higher gain can be achieved by block-planting seedlings from families with high genetic values, rather than mixing all seeds from a given seed orchard (IX; Li et al., 1999). The family block system redistributes the offspring of the orchard, but it does not much reduce the genetic diversity found in the orchard because the paternal contribution is uncontrolled (Duzan and Williams, 1988). Genes from all parents can thus be represented by the use of half-sib families. Even if seeds were collected from the single mother, the effective number (status number) of the seeds would be close to four (IX). Risk of cataclysmic disaster from pests may be slight because genetic variability within a given half-sib family block is still quite large (Bishir and Roberds, 1999). A variation on selective harvest is to collect equal numbers of seed from each parent (I, III, X; Bila, 2000). When each genotype contributes equally to the progeny as a mother parent, gene diversity will improve depending on the levels of fertility variation and correlation between gender fertilities (VI). For the complete equal seed harvest, however, the size of the collection will be determined by the parent having the lowest fertility, resulting in a great loss of seed production. A tradeoff among genetic gain, seed production and gene diversity (status number) can be achieved when constraints are made on the selected fraction of ranked parents (II and X). By controlling maternal contribution, high status numbers could be obtained, although accompanied by some loss of seed production (X). For a tradeoff between seed production and gene diversity, expected seed production (y) is based on the probability density power function when the genotypes are ranked according to fertility, F(x)= xa as xj a 1 a 1 y ax a1dx ax j (1 x j ) x j x0a ax j (1 x j ) a [11] x0 where a is a parameter; x0 is the minimum contribution (lower bound), meaning that seeds are not collected from parents with very low fertility because it is 29 regarded to be expensive; and xj is the maximum contribution of individuals on ranked parental fertility (upper bound; fertility balancing). This results in an equal amount of seed collected from parents with fertility higher than the upper bound, to avoid over-representation of the most fertile ones (see Fig. 6). If there is a strong negative correlation between breeding value and fertility of individuals, this procedure is not attractive, as gain will be lost. With a positive correlation, however, the balancing may give desirable genetic quality in the seed crop. 2. Genetic thinning In many seed orchard programs, orchard parents are evaluated in genetic tests and those that prove to be genetically inferior are removed, i.e., genetic thinning or roguing. In genetic thinning, selection is applied simultaneously to both seed and pollen parents with the same intensity. Genetic gain by roguing only will then give G (1 M )i( Nm , N ) A MC [12] There is no practical way in which an orchard can be designed to provide for roguing, as one does not know ahead-of-time when clones are to be removed. As a consequence, true roguing tends to result in a somewhat uneven distribution of trees in the orchard (VII). If the number of clones to be removed is small, in comparison to the total number of trees that need to be thinned to provide adequate spacing, the problem is not too serious. Usually, some holes and clumps are unavoidable. Roguing is also a management technique that can improve the genetic quality of seed and increase seed yield due to better crown development. Most concern about the genetic thinning is the loss of genetic variability in orchard crops after roguing, because roguing is a kind of family selection. However, simulated genetic thinning in the orchard did not drastically reduce genetic diversity (heterozygosity), even when as many as 50% of orchard clones were rogued (I; Stoehr and El-Kassaby, 1997). Savolainen and Kärkkäinen (1992) also reported that the effect of random genetic drift on depleting heterozygosity in seed orchards was low. Most parents with high fertility can produce well for successive years, but some parents do not (III, V; Goddard, 1964; Eriksson et al, 1973). It could thus be possible to control fertility during roguing (Hodge and White, 1993) or seed collection (X), so that sufficient genetic variability would be maintained. The overriding factor in roguing a seed orchard is economics. The potential loss in seed production needs to be balanced against the expected improvement in quality (X). Roguing gives an initial seed loss, but in the long run the remaining 30 trees will produce more seeds and the accumulated seed loss may not be so large. The best roguing solution may be obtained by weighting each clone according to its seed production and the percent of superiority of its seed. For example, a clone producing 100 kg of seed annually that is 10% better than nursery-run seed is equal to a clone producing 200 kg of seed that is only 5% better. Both are inferior to a clone producing 75 kg of seed that is 20% better. It varies with the particular circumstances whether genetic quality of the seed is a more important goal than its quantity, or vise versa. 3. Combined method The former alternatives can be combined, i.e., genetic thinning first, followed by harvesting the best clones. In this alternative, parents that serve as pollen source are the total number of parents remaining after thinning. Cones are then harvested from more restrictively selected parents with higher breeding values from the remaining part. Selection thus occurs twice with different intensity. The first is simultaneous selection against pollen and seed parents at the time of thinning, so that the selection intensity is derived from the initial number of parents. The second is more intensive selection against seed parents for the selective cone harvest. The genetic gain is thus calculated from the average of selection as G i ( Nm ,N ) (1 2M )i( N f , N ) A 2 MC [13] Calculations of relative total gain from orchard management (or breeding methods) may change greatly with the many factors (Namkoong et al., 1966). Not all factors can be kept comparable for a fair comparison of the management alternatives. Many factors, such as heritability, gender fertility correlation, cost and time, should also be considered to decide which tactics would be more effective in the management of seed orchards (e.g., Danbury, 1971). The foregoing rates of genetic gain are based on the gain obtained at the end of an operation or cycle, which are the figures most commonly of interest to tree breeders. On the other hand, seed orchard managers may wish to determine the value of the orchard on the basis of realized gain over the lifetime of the seed orchard, as in Figure 1. 4. Genetic parameters Genetic gain is, in a statistical sense, calculated from simple linear regression, y = bx. The regression coefficient, b (e.g., heritability) is the ratio of the covariance between the predictor variable x (e.g., selection differential) and the predicted gain (y) to the variance of the predictor x as b = cov(x,y)/var(x). The covariance is composed of two parts: genetic differences and sampling error (Namkoong et al., 1966). It is the genetic covariance between the breeding value of parents and the 31 expected growth of trees grown from seed orchard seeds. The covariance between them is, in general, controlled by purely genetic and G x E interaction effects. Genetic models are often complicated; so the composition of cov(x,y) or var(x) is not clear and hence the heritability is not interpretable accurately (see Namkoong et al., 1966 for summarizing). The other errors in genetic testing are faulty or inaccurate procedures. In publications V and XI, the analyses of variance indicated that there were statistically significant differences among orchard clones in flower production. Broad-sense heritability estimates (H2) also implied that the reproductive output attributes were under genetic control (Matziris, 1994; Schmidtling, 1983). The correlations between female and male strobilus production were all weakly positive for the four years studied in a Pinus densiflora seed orchard, but they were positive or negative for five successive years in a Pinus thunbergii orchard. It has been reported that there were no significant correlations between volume growth and cone production (Skroppa and Tutturen, 1985; Byram et al., 1986). The relationship between fertility and progeny growth rate may thus be too weak to use as a criterion for genetic thinning (I). Parents with desirable genetic values might be penalized. Schmidtling (1981) and Nikkanen and Velling (1987) reported negative genetic correlations between flowering and growth. In their studies, correlations between mid-parent fruitfulness and progeny growth rates were negative, with the exception of a positive correlation with diameter. It is generally suggested that taller trees allocate much of their energy to vegetative growth rather than to reproductive growth, so the most fruitful parents may produce slower-growing progeny. If there were indeed negative correlations, roguing based on flower production would result in a loss of volume growth. Sexual characters (e.g., fertility, fruitfulness) are peculiar traits in comparison to other traits (e.g., growth rate, gum yield) that are usually included in a breeding program. Seedlings with high seed production potential may not be needed for commercial forest planting, because it is foreseen that the vast majority of planted stands are to be replanted artificially (Varnell et al., 1967). Desirable orchard trees should still be heavy seed producers. It may be speculated that seed orchard environments and stand environments are different, so that trees flowering well in seed orchard environments may not necessarily produce progeny that flower well in the field. But, investigations are difficult to conduct because of the long lifespan of trees. The best might be to have trees which are fertile only in seed orchard environments and not otherwise, and this might be possible with a particular treatment regime (e.g., chemical floral induction) in seed orchards. Recently, new selection methods have been proposed in which both breeding values and genetic relationships among members in the selected population are considered. Brisbane and Gibson (1995) regarded the balance between genetic gain and relatedness (gene diversity) as important to achieve high genetic gain 32 with minimal inbreeding. Similarly, low group coancestry combined with optimized genetic gain was proposed by Lindgren and Mullin (1997), and by Olsson et al. (2000). Gene diversity (status number) Concerns over the reductionist nature of the domestication of forest trees focus on the possibility of genetic erosion. There are several steps in the forest-tree domestication process in which genetic variation could be reduced if sufficient safeguards are not considered (Stoehr and El-Kassaby, 1997). The steps are phenotypic selection, breeding (tree improvement cycle), seed and seedling production. Systematic monitoring of the various steps of the domestication process should be identified (X; El-Kassaby and Namkoong, 1995). Phenotypic selection of plus trees has a little impact on the reduction of gene diversity in seed orchard populations. Seed orchards harbor levels of genetic variation that are similar to, or even greater than, those found in their naturalpopulation counterparts (Knowles, 1985; Cheliak et al., 1988; Bergman and Ruetz, 1991; Chaisurisri and El-Kassaby, 1994; Williams et al., 1995). Other less obvious sources of genetic erosion could occur during the seed and seedling production phases. El-Kassaby and Thomson (1996) reported that the genetic variation of seedling crops was reduced due to the unintentional, directional selection through nursery management practices. The status number of the orchard parental genotypes is the same as their census number, if they are assumed to be unrelated and non-inbred. Status number and group coancestry are used as measures of what happened since tree improvement started. Of interest is a possible relationship among trees from various geographic origins, and between the origin and the seed production of clones (XI; Goddard, 1964). The main criteria for plus-tree selection are to select one, or a few widely separated, vigorous, healthy and well-adapted trees within a stand. The criteria are hard to fulfill in practice, so that there could be a few relatives and some relatedness among selected trees. However, such deviation of criteria could be ignored because a large number of parents have generally been used in firstgeneration seed orchards. In seed orchards, status number is best described as the status number of orchard crops (Lindgren and Mullin, 1998). Status number of the seed orchard itself is equivalent to the status number of the orchard crops only when parents are unrelated, non-inbred and have equal fertility, and when there is no contamination by outside pollen sources. 1. Relatedness and group coancestry When orchard parents are unrelated and non-inbred, group coancestry of orchard crops is calculated only from self-coancestry (i.e., = 0.5/N). Expected status 33 number of seed crops will thus be the same as the census number (N). Under such conditions, group coancestry of orchard crops can also be calculated from the contribution of parents (pi) as N 0.5 pi2 [14] i 1 If all parents are assumed to be non-inbred, indicating that all self-coancestry equals 0.5, but are related each other, ij, group coancestry becomes 0.5 ( N 1) N N Ψ ij i 1 i j N [15] Group coancestry (status number) depends on the size of the orchard population (Fig. 4), since group coancestry includes the repeated sampling of the same gene (Rosvall, 1999). Status number decreases as relatedness among parents increases. Relative status number 1.00 N = 10 N = 20 N = 100 0.75 0.50 0.25 0.00 0.00 0.05 0.10 0.15 0.20 0.25 Relatedness between parents Figure 4. The relationship between relatedness and relative status number depending on the size of orchard population. Equal fertility and no pollen contamination are assumed. The decrease of relative status number is profound in orchards with many parental genotypes (Fig. 4), reflecting that status number cannot be very large if population members are related. At a given level of relatedness, orchards with fewer genotypes will retain higher relative status number. Gea et al. (1997) 34 reported, using stochastic simulations, that breeding schemes with small, disconnected groups were slightly more efficient in preserving status number through a large number of generations than those with large groups. However, when breeding programs are started with very small groups, or with status number of less than 10, there will be lower expected genetic gain and potential problems with inbreeding (VII and IX). Group coancestry for the gamete gene pool of the seed crop is calculated based on the contribution and relatedness of orchard genotypes, and pollen contamination as (I; cf. Lindgren and Mullin, 1998) N N i 1 j 1 ( f i (1 2M )mi ) ( f j (1 2M )m j ) ij [16] where fi and mi are the female and male contributions of genotype i (sums up to 0.5, respectively); fj and mj are those contributions of genotype j; and ij is the coancestry between genotypes i and j. Here, mi and mj refer to the male contributions in the absence of gene migration (M). Theoretically, the group coancestry () can vary from a minimum of one-eighth the census number to a maximum of one. And thus, the status number (Ns) has inversely the range from the maximum of 4N to the minimum of 0.5 as 0.125N < < 1 and inversely 4N > Ns > 0.5 When there is one completely inbred genotype and only selfed seeds are collected from it, the coefficient of inbreeding will be one. The group coancestry of selfed seeds from the pure line will also be one and Ns = 0.5 (IX). If a tree mates equally with unrelated and non-inbred pollen sources, the seeds are all half-sibs and the group coancestry of a large amount of seeds from the same seed parent approaches 0.125 and, hence, Ns approaches four (I, IX; Lindgren and Mullin, 1998). 2. Fertility and ramet variation Maximum gene diversity of seed orchard crops can only be attained when all parents contribute equally to the gamete pool (see Fig. 5 and Table 2). This assumption is virtually never fulfilled and it is commonly observed that a small portion of orchard parents contributes a disproportionately large amount to the orchard crop (El-Kassaby et al., 1989a; El-Kassaby and Cook, 1994). The unequal contribution leads to an accumulation of genetic relatedness and the loss of gene diversity in seed crops (II, III, IV and XI). 35 Balanced and unbalanced contributions from parents in different orchard populations are illustrated in Figure 5. This might also represent different flowering years. The results are presented in Table 2. In population A, all parents contribute equally, so there is no fertility variation and the census number of the orchard is exactly the same as the status number of orchard crop. Due to unequal gamete contribution, the status numbers of orchard crops from B and C decrease 29% and 47%, respectively, when compared to the parental census number (Table 2). Population C shows the typical unequal contribution that was found in study IV ( = 2: CV = 100% in fertility). Orchard population A (equal contribution) Orchard population B (unequal contribution) Orchard population C (typical contribution) Figure 5. An illustration of the parental gamete contribution in seed orchard populations with five unrelated individuals Table 2. Fertility variation (CV, ), status number (Ns) and relative status number (Nr) for the populations illustrated in Figure 5 where parents are unrelated and non-inbred Orchard pop. No. of trees CV Ns Nr A 5 0 1 5 1.00 B 5 0.63 1.4 3.6 0.71 C 5 0.95 1.9 2.6 0.53 In paper III, an effective number of parents is proposed, based solely on the fertility variation. Effective numbers of seed and pollen parents can be estimated separately (VI; Roberds et al., 1991). Inherent variation in male flowering is large, but the effects are seldom quantified. Male strobilus production is more difficult to assess than female strobilus production, because catkins are numerous and usually do not persist after pollen shedding (Schmidtling, 1983). If there is no correlation between maternal and paternal fertilities, the sibling coefficient () in an orchard is calculated with pollen contamination (M) as (III, VIII; cf. Nikkanen and Ruotsalainen, 2000) 36 f (1 2M )mi Ψ N p N i i 1 i 1 2 0.25 f (1 2M ) 2 m 0.5(1 2M ) N 2 i 2 N [17] where N is the number of genotypes contributing to the gamete pool; pi is the contribution of genotype i; f and m are the fertility variations for female and male parents, respectively; and M is the gene migration due to pollen contamination (i.e., half the pollen contamination). When there is a correlation between female and male fertilities, the correlation coefficient (r) should be considered for estimating total fertility variation in the seed orchard (VI). The sibling coefficient () can be calculated as Ψ f (1 2M ) 2 m 2(1 2M ) 2r (1 2M ) ( f 1)( m 1) 4(1 M ) 2 [18] If there is a perfect positive correlation (r = 1) between gender fertilities, the sibling coefficients for maternal, paternal and total fertility all are the same ( = f = m). On the other hand, if there is a perfect negative correlation (r = -1), the sibling coefficient for total fertility equals one ( = 1) and f = m. Most studies have shown that male and female flowering are weakly, positively correlated (II, V; O’Reilly et al., 1982; Schoen et al., 1986; Kjær, 1996; Nikkanen and Velling, 1987; Nikkanen and Ruotsalainen, 2000), or not related (III, XI; Savolainen et al., 1993). Negative genetic correlations between female and male flowering have been found in slash pine (Schultz, 1971) and in Scots pine (Savolainen et al., 1993). This supports the central assumption of the theory of sexual allocation: given a fixed total quantity of resources available, there should be a negative correlation between female and male allocation. This negative relationship could also be an important consideration in seed orchard management; if the parents are rogued with strong intensity, some of the best pollen sources may be eliminated (Danbury, 1971; O’Reilly et al., 1982; Schmidtling, 1983). This in turn would narrow the genetic base and perhaps reduce the contribution of some of the better genotypes. One way to reduce the loss of gene diversity due to fertility variation is to restrict the parental contribution to the next generation (VIII; Wei, 1995; Bila, 2000). This restriction is more likely to be applied to the maternal contribution. By keeping the contribution equal among genotypes, genetic relatedness is minimized and gene diversity is maximized in the orchard crop (Lindgren et al., 1996). When flower phenology is a concern, removing parents with unusually early or late flowering from orchards may also be an effective way to maintain 37 high genetic gain and gene diversity, and a means to reduce pollen contamination and selfing (Xie and Knowles, 1994). Equal seed harvest is often proposed to mitigate the effect of unbalanced contribution among parents in stands and seed orchards (III, VI, X; Schmidtling, 1983; Bila, 2000). Equal seed harvest means that female fertility is kept constant and thus no correlation between female and male fertilities. When an equal amount of seed is collected from each parent, the sibling coefficient is a function of male fertility variation (m) and pollen contamination (M) as Ψ (1 2M ) 2 m 4M 3 4(1 M ) 2 [19] In paper VI, I evaluated how much better was equal seed harvest, compared to seed collection where both maternal and paternal fertilities vary. The magnitude of equal seed harvest depended on fertility variation and correlation strength between genders. In general, it was concluded that collecting the same amount of seeds across the population reduced considerably relatedness of seed crops, and this option was an effective way of maintaining high gene diversity. Equal seed collection among parents could also be considered to apply in germplasm collection and when establishing stands for ex situ gene conservation (X). Sexual asymmetry was investigated in study V by means of a maleness index, Mi = mi/(fi+mi) (cf. functional gender in Devlin and Ellstrand, 1990). A maleness value near 0.5 indicates that female and male fertilities are nearly equal, while a value near one means that the individual transmits genes to the seed crop predominantly through pollen. Femaleness equals 1-Mi. It was shown in study V that orchard parents commonly differed in their genetic contributions to seed crops through pollen and ovules, so that fertility estimates based solely on one gender might be misleading. As mentioned earlier, harvesting or mixing equal amounts of seeds from each parent is suggested as a means to mitigate the imbalance of gamete contributions among parents (III, VI, X; Bila, 2000). Completely equal seed harvest will discard most of the crop, because equalizing of female fertility will be set to the least-fertile parent, and translate to a substantial loss of seed production. It is, of course, impossible to apply when there is an individual with zero fertility. In study X, a tradeoff between seed production and gene diversity was achieved by equalizing maternal contributions from over-represented parents and discarding seed from the least-fertile parents. By controlling female fertility, high gene diversity could be obtained while an acceptable amount of seed was produced. Revised results from study X are shown in Figure 6. When all seeds were collected, the status number was 149. By balancing with constraints of x0= 0.1 38 and xj= 0.9, the status number increased as 183 and the loss of seed production was only 13% (i.e., 87% of the total crop was collected). This balancing between gene diversity (measured by status number) and seed collection will be more important in genetic resource conservation programs. 0.025 Observation Balancing Individual fertility 0.020 All seed production x 0=0 & x j =1 0.015 Balancing x 0=0.1 & x j =0.9 0.010 0.005 0.000 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 Fraction of ranked parents (x 0 & x j ) Figure 6. Tradeoff between gene diversity and seed production by controlling female fertility in the collection of Corylus avelana L. from native Danish populations. Balancing improved fertility variation and thus increased status number. Discarding seed by constraints will increase Ns, but may decrease the likelihood of retaining rare alleles present in the population. Loss of gene diversity caused by selection is quantified by heterozygosity or reduction in allele numbers (Allendorf, 1986). However, the number of alleles remaining after selection is important for the long-term response, while heterozygosity provides a good measure of the response in diversity to selection immediately (see formulae 23 and 24). Fertility variation is higher in stands than in seed orchards (IV). Differences in fertility were usually higher during poor flowering years and in young populations. Forecasts of the effect of tree breeding and conservation operations require information on fertility variation. In most cases, the information does not exist or is highly unreliable. For predictions concerning objects that are neither juvenile nor characterized by poor flowering, it is suggested in paper IV as a rough generalized heuristic rule that equals 2 (CV in fertility = 100%) for seed orchards and = 3 (CV = 140%) for stands. These values are a little higher than values actually indicated by observations, but after truncation they come out 39 about right. That they are a little high may mean an extra safeguard for gene diversity, which seems prudent. They are recommended for use in calculations where other information does not exist (probably in most cases). Generally speaking, therefore, the effective number is about 150% larger in seed orchards than in stands of the same size. In seed orchards, trees are widely spaced with well-developed crowns and limited height. Genetic thinning is done to eliminate families or clones with low genetic values and those with low flowering and fruiting abilities (Varghese et al., 2000). Topping and pruning are performed to maintain a short, wide crown, encouraging the growth of lateral branches and thus increasing the number of potential flower and seed production sites (Ho and Schooley, 1995). Irrigation, fertilizers and plant growth regulators may be used to induce and enhance flowering (Setiawati and Sweet, 1995; Owens, 1995). The effects of these practices are likely to make trees more similar, which correspond well with the lower fertility variation in seed orchards compared to stands (IV). The sibling coefficient is increased with unbalanced parental contribution to the progeny. When the sibling coefficient equals 2, it means that relatedness and inbreeding of seed crops will build up over generations twice as fast as the reference population (VIII). The “20:80 rule” is often quoted (Anon., 1976) to describe the imbalance, asserting that 20% of trees in a seed orchard often produce 80% of the cone crop. This rule has been proven in many orchard species (Eriksson et al., 1973; Griffin, 1982; Schmidtling, 1983; El-Kassaby et al., 1989a; El-Kassaby and Reynolds, 1990). Understanding such imbalance has implications for selective treatment, prediction of realized genetic gain, expected gene diversity of orchard crops, and crop prediction models (Byram et al., 1986). Parental balance curves have also been used widely (V; Griffin, 1982; ElKassaby and Reynolds, 1990; Adams and Kunze, 1996). They are informative in determining the present contribution of the various clones to either female or male strobilus production, and in identifying the high and low producers (ElKassaby and Askew 1991). Besides fertility variation, the variation in the number of ramets among parents will also affect the genetic composition of orchard seeds. In study VII, a survey of 255 seed orchards indicated that there was a considerable ramet variation among clones. The coefficient of variation (CV) in ramet number was about 60%. The average size of orchards was about 12 ha and the average total number of ramets was 3,798 per orchard (Table 3). Thus, orchards had an average density of approximately 320 ramets per ha. On average, the surveyed orchards were 23 years old. Table 3. Average clone number (N), total numbers of ramets, CV for ramet number and relative effective number (Nr= 1/) in first-generation clonal seed orchards 40 Orchard location Species Number of SO N Finland P. sylvestris 176 139.0 4,137 0.469 0.81 Picea abies 25 75.4 3,182 0.602 0.74 P. koraiensis 13 70.4 2,689 0.852 0.60 P. densiflora 8 94.0 5,454 0.752 0.65 P. sylvestris 22 79.8 4,614 0.503 0.80 Picea abies 11 71.2 2,713 0.382 0.86 88.3 3,798 0.593 0.74 Korea Sweden Average Number of CV for ramet ramets/SO number Nr Ramet variation found in study VII was mainly caused by factors such as graft availability, incompatibility, graft replacement, and biotic or abiotic injuries. Unusual weather can significantly delay a seed orchard program and also cause variation in numbers of ramets (Byram et al., 1998). Moreover, intentional variation often occurs in later stages. As seed orchards get older and their associated progeny test data become available, further reduction in numbers of parents is expected through the genetic thinning of poor parents. There were large differences in the numbers of clones and ramets between unthinned and thinned orchards, and also between young and mature orchards (VII). The census number of clones (N) in surveyed seed orchards was generally high and could give information on gene diversity. The effective number (Ns) will be more informative in advanced-generation seed orchards where parents will be more closely related. Also, the effective number estimated from ramet and/or fertility variation can probably give information for the deployment of genetically improved stock for capturing high gene diversity (maybe also gain) and the development of seed-trade guidelines. It is thus recommended in study VII to use the concept of effective number of parents for the certification of seed orchards and their crops following OECD scheme. When estimates of orchard gain are based on tests of open-pollinated progeny, it is often assumed that parental fertilities are equal and that pair-wise mating probabilities are identical. However, the violation of such assumptions, particularly where a small number of parents contribute most of the gametes to the seed crop may lead to inaccurate estimates of gain (Schoen and Stewart, 1987). Under such a system of mating, the actual response to selection may also be higher or lower than expected, even when accurate estimates of GCA have been obtained (Griffin, 1982). 41 In mature orchards, seed production is more evenly distributed among parents in good or intermediate crop years (V; Eriksson et al., 1973; Byram et al., 1986; Kjær, 1996). Maximum panmixia is thus more readily achieved in good or moderate flowering years than in poor years (O’Reilly et al., 1982; El-Kasaby et al., 1989a; Chaisurisri and El-Kassaby, 1993). It was also found for the Pinus densiflora seed orchard reported in study V that parents contributed more equally to the seed crop and that status number was higher in moderate flowering years. Parental ranks also varied year-to-year, mainly among parents with intermediate fertility. Variation in fertility has important implications in breeding (Griffin, 1982; Xie and Knowles, 1992; El-Kassaby, 1995) and conservation programs (Sedgley and Griffin, 1989). Differences in gamete contributions among orchard parents influence the genetic composition of offspring by over-representing the most productive genotypes (V; Kjær, 1996). Fertility variation represents fertility selection that changes gene frequency and reduces effective number and viability. Genetic drift and inbreeding will occur more rapidly than expected from the census number. Special attention should thus be given to fertility variation in order to avoid rapid genetic erosion in gene conservation or breeding programs. Fertility in some studies was estimated by counting the number of reproductive structures; however, there may be other factors, such as separation distance, phenology, pollen dispersal, genetic incompatibility and local weather conditions, playing an important role, especially for the male fertility. The effect of the localization of individual clones on the selfing and genetic composition of orchard crop is generally neglected (Gömöry et al., 2000). In seed orchards, repetition of parents with several times (often 30-40 times in clonal seed orchards, VII) and differences in floral phenology are supposed to overlay the effect of distance among mates as a determination of mating success (El-Kassaby et al., 1984; Askew, 1988; Adams and Birkes, 1991; Matziris, 1994). Separation distance may significantly affect male mating success in Douglas-fir seed orchards (Burczyk and Prat, 1997). If there is high proportion of mislabeled or incorrectly planted individuals, the distance might become an even more important factor (Gömöry et al., 2000). There have been many reports that there is a positive correlation between the number of seed cones and the proportion of filled seeds (Reynolds and ElKassaby, 1990; Chaisurisri and El-Kassaby, 1993; El-Kassaby and Cook, 1994; Kjær and Wellendorf, 1997), and that pollination success is a positive function of flowering production (Schoen and Stewart, 1987; Allison, 1990). Schmidtling (1983) reported that the genetic correlation between female flowers and sound seeds were strongly positive, indicating that clones with produced more female flowers were the ones that produced more seeds. The simple counting of numbers of strobili can thus give a reasonable indication of the unequal contribution among parents in seed orchards (II, III, IV and XI), notwithstanding that heavy 42 flowering may not always result in a heavy seed crop, especially in natural stands (Koski, 1980a). Synchronization of pollen shedding and flower receptivity is important for gamete pairing and influences the impact of foreign pollen, especially in seed orchards with large numbers of parents (Askew, 1988; El-Kassaby et al., 1984). On the other hand, the effect of reproductive phenology on the genetic composition was found to be negligible in black spruce (O’Reilly et al., 1982) and radiata pine seed orchards (Griffin, 1982). Variation in fertility within all investigated orchard populations could be attributed to genetic and environment effects, since both are confounded, although reproductive traits (e.g., flowering, fruiting, seed production) are generally moderately strong genetic control (V, XI; Schmidtling, 1983; Byram et al., 1986; El-Kassaby and Cook, 1994; Fries, 1994; Kjær, 1996). Fertility variation can be studied by marker genes, but such techniques have not been sufficiently accurate, nor have data sets been large enough for quantitative analysis. If a clone at one locus carries one or two alleles unique or rare to the orchard, it may then be possible to trace the contribution of that particular clone to the seed crop (Rudin and Lindgren, 1977). In the absence of such unique alleles, the use of open-pollinated seeds collected from a clone and counting the plants arising from these can document cross-pollination (Kormut'ák and Lindgren, 1996). In addition, information on the occurrence of selfing can be obtained. Hormonal treatments (e.g., gibberellins) are recommended when reproductive induction and synchrony are fundamental prerequisites for the production of seeds reflecting the gene diversity represented in the orchard (Ross, 1978; Pharis et al., 1980; Ross et al., 1985; Wheeler et al., 1985). Some hormones (e.g., GA 3) have a negative or no affect on male flowering (Chalupka, 1980; Eriksson, 1996), depending on environmental conditions and hormone concentration. Physical treatments (e.g., root pruning, girdling) are sometimes recommended, but these would often harm the treated trees for long-term seed production (Eriksson, 1996). Supplemental mass pollination (SMP) is defined as the broadcast application of pollen to non-isolated female strobili (Bridgwater and Trew, 1981). SMP has been proven to be a vital tool for improving reproductive success, on average of 20%, due to the reproductive phenology displacement between male and female strobili, and more uniform parental contribution in Sitka spruce (Franklin, 1971a; El-Kassaby et al., 1989b; Chaisurisri and El-Kassaby, 1993) and Scots pine seed orchards (Yazdani et al., 1986; Eriksson, 1996). SMP can produce more and better-filled seeds compared to those produced by normal wind pollination (ElKassaby and Reynolds, 1990). An overhead cooling system with SMP on earlyand late-reproductive phenology classes in moderate and good flowering years is 43 effective in disrupting the synchrony, in minimizing pollen contamination and inbreeding, and in reducing seed loss due to insect damage in Douglas-fir seed orchards (Fashler and Devitt, 1980; El-Kassaby and Ritland, 1986b; Fashler and El-Kassaby, 1987; El-Kassaby et al., 1990; El-Kassaby and Davidson, 1990). 3. Pollen contamination Pollen contamination is a main source of gene migration into seed orchards, which is probably the most serous impediment to the genetic quality of orchard crops (Adams and Birkes, 1989). It is hard to avoid pollen contamination in practical orchard management, even though wide isolation strips are often used to reduce background pollen (Wheeler and Jech, 1986). Gene dispersal through pollen influences levels of inbreeding and effective population size (Adams et al., 1992). Numerous studies have been concluded that considerable pollen contamination occurs in seed orchards (Table 4). It seems that many of the seeds from seed orchards will have unknown fathers from outside the orchard. Although contamination levels are high, the accuracy of many estimates is in doubt (Stewart, 1994). For estimating background pollination, it is evident that orchard size, shape, pollen production and phenology must be important. For example, Yazdani and Lindgren (1991) reported that pollen contamination was higher in the windward part of the orchard and lower in the center. Understanding the underlying process of pollen movement from outside to seed orchards is also important (Di-Giovanni and Kevan, 1991). Levels of pollen contamination are of concern because seed resulting from fertilization by alien pollen is expected to have lower genetic gain than seed fertilized by orchard pollen (I). The contamination affects only the male side, and thus considerably more than half of the gene in the orchard crop will have a seed orchard origin. Low levels of pollen contamination could actually increase gene diversity of orchard crops (IX; Lindgren and Mullin, 1998), but high levels could either increase or decrease gene diversity, depending on the source of contamination (Adams and Kunze, 1996). Reduction in genetic gain may even be worse if contaminating pollen comes from trees that are poorly adapted to the intended planting sites. Orchard managers should have a better understanding of how various parameters such as orchard size, age, composition and degree of the isolation influence levels of contamination, and the degree to which pollen management tactics are effective in limiting pollen contamination (Wheeler and Jech, 1986). Table 4. Estimates of background pollination (%) in seed orchards Species Larix decidua 44 Background pollination 5 References Gömöry and Paule, 1992 Larix europaea x L.leptolepis 1.0-6.7 Häcker and Bergmann, 1991 Pinus elliotii 83.5 Squillace and Long, 1981 Pinus sylvestris 2 38 21-36 26-33 30 6-20 69-74 11 48 26 36-60 Müller-Starck, 1982 Nagasaka and Szmidt, 1985 El-Kassaby et al., 1989b Harju, 1995 Wang et al., 1991 Pakkanen and Pulkkinen, 1991 Yazdani and Lindgren, 1991 Paule and Gömöry, 1992 Harju and Nikkanen, 1996 Friedman and Adams, 1981 Friedman and Adams, 1985b Picea abies 10-17 Paule et al., 1993 Picea mariana 31.4 Caron, 1987 Pseudotsuga menziesii 15 5-15 29-52 0.2 12 22-56 50 49 34 a Fashler and Devitt, 1980 Clare, 1982 Smith and Adams, 1983 El-Kassaby and Ritland, 1986a El-Kassaby and Ritland, 1986b Wheeler and Jech, 1986 Adams and Birkes, 1989 Adams et al., 1997 Xie et al., 1991 Pinus taeda a ) clone bank. Note that the author has not corrected all observations to make them comparable and accurate. The main factor that detracts from the usefulness of seed from orchards is background pollination (Pulkkinen, 1994). When migrating pollen contributes effectively to pollination and fertilization, it may implicate some genetic consequences in the adaptation process of forest trees (Koski, 1970; Lindgren, 1975). If the seeds are hybrids pollinated by the surrounding stands, the resulting seedlings may have poor chances for survival in the areas from which the parents originate because adaptation depends on the genotypes of both parents (Mikola, 1993). Stoehr et al. (1994) point out that if pollen contamination is not prevented, the faster-growing seedling sired by background pollen may be preferentially selected during culling in the nursery and out-planted on sites to which they are maladapted. Pollen contamination in seed orchards involves a long-distance transport of pollen (Chalupka, 1998). The outside pollen may also influence genetic variation in the seed-orchard progeny (I; Müller-Stark, 1982). 45 In general, some female flowers are receptive before the tree sheds pollen. Female receptivity often occurs before pollen shedding in young seed orchards. In this case, most pollen comes from outside the seed orchard. On the other hand, Yazdani et al. (1995) reported a situation where pollen production in the orchard commenced a little earlier than in surrounding stands, because more sunshine reaches the ground in a seed orchard as the canopy is more open and the trees shorter in the seed orchard than in stands. There may be insufficient pollen at early dates and female strobili may remain receptive until the orchard pollen production commences. At later dates, the surrounding pollen cloud from local sources may be more intense relative to the seed orchard pollen cloud. Harju and Nikkanen (1996) reported that background pollination varied during the pollination season. The genetic quality of seeds from orchards is increased by genetic thinning or by establishing advanced-generation seed orchards, but the full genetic potential is never reached because of pollen contamination (Bridgwater et al., 1998). DiGiovanni and Kevan (1991) estimated that a genetic gain of 8% from selection was reduced to 6% in the next generation if 50% of the pollen originated outside the orchard. Due to pollination from foreign pollen, the number of alleles at allozyme loci in orchard crops is clearly higher than in the orchard parents (Harju, 1995). Numerous reports, for a variety of conifer species, indicate that even in mature seed orchards with heavy pollen production, levels of pollen contamination could exceed 30-40% (Table2). Furthermore, the relationship between levels of pollen contamination and within-orchard pollen production is not clear. Smith and Adams (1983) predicted that as the orchard matured and pollen production within the orchard increased, pollen contamination would diminish in a Douglas-fir seed orchard. This is not the case in Scots pine seed orchards (Harju, 1995; Pakkanen and Pulkkinen, 1991). Contamination by extraneous pollen should be avoided. Possible methods used to reduce the contamination include: 46 geographical isolation by establishing orchards at the margin or slightly outside range of species (Sarvas, 1970; Hadders, 1972) or at a different elevation (Silen, 1963); reducing the frequency of outside pollen by the use of SMP (Denison, 1973; Bridgwater and Trew, 1981; El-Kassaby and Ritland, 1986b), establishing larger orchards (Wright, 1953), and/or surrounding orchards by isolation zones (Wright, 1953; Squillace, 1967); and physiological isolation through phenological manipulation, e.g., by the use of water-spray cooling treatments (Silen and Keane, 1969; Fashler and Devitt, 1980; El-Kassaby and Davidson, 1990). There are two main methods of estimating levels of background pollination. In the “migration model”, migration changes the allelic frequency of the recipient population towards that of the source population. If there are appreciable differences between the source and recipient population, the migration can be estimated based on neutral genetic markers or progeny tests. In the “finite population model”, the finite orchard population produces only a limited number of all the possible gamete genotypes, while the infinite surrounding populations are expected to produce all possible combinations of alleles present (Savolainen, 1991). Pollen genotypes that cannot be produced in the seed orchard are detected as background pollination. For contamination levels in seedling seed orchards, estimation based on differences among gene frequency estimates derived from single-locus outcrossing pollen, ovule and outside seed is suggested by El-Kassaby and Davidson (1990), and Wheeler and Jech (1992). In clonal seed orchards where the number of within-orchard genotypes is detected by the number of clones, the method that relies on detecting unique multilocus gametes is recommended by Smith and Adams (1983). Stoehr et al. (1998) evaluated pollen contamination and SMP efficacies using a genetic marker on the paternally inherited chloroplast genome, and proposed to use DNA makers for assessing orchard mating dynamics and management efficacies in Douglas-fir seed orchards. If significant differences among maternal (ovule), paternal (pollen), and progeny pools are observed, it indicates that the pollen pool includes some pollen from outside the orchard. On the other hand, if there are no significant differences among the three gene pools, it implies similarity between the allelic frequencies within the orchard population and in the outside pollen source. This similarity makes the detection of contamination based on unique alleles or differences in allele frequencies very difficult (El-Kassaby et al., 1989b). 4. Different sex ratio While most animal species reproduce by outcrossing and have separate sexes, plants display a wide variety of sexual systems ranging from hermaphroditism and complete self-fertilization to dioecism and complete outcrossing (Kärkkäinen, 1994). Avoidance of self-fertilization is the key factor shaping the evolution of reproductive structures in plants (Darwin, 1877). Darwin believed that separate sexes helped to avoid self-fertilization and sexual allocation might also be an important factor. That is evidently true in forest tree populations and maintains high gene diversity. Most economically important tree species have a mixed mating system, predominantly outcrossing with some selfing, which makes the assay of gene diversity difficult. In papers I and IX, formulae were derived to calculate gene diversity (status number) based on sex ratio, pollen contamination and fertility variation in seed 47 orchard populations. The status number (Ns) for the seed crop is described, considering the number of female parents (Nf), male parents (Nm) and parents that are functioning as both female and male at the same time (Nfm) by NS 4N f Nm N m (1 2M ) 2 N f 2(1 2M ) N fm [20] This is equivalent to the effective number given by Frankel and Soulé (1981) and by Falconer and Mackay (1996) when there is no gene migration, i.e., the status number becomes Ns = 4*Nf*Nm/(Nf + Nm) in dioecious species. If the sexes are not equal, Ns is less than N. On the other hand, Ns is the same as N when the sexes are all equal (Nf = Nm = Nfm), as under an idealized situation. Note that Nfm cannot be larger than the smaller of Nf and Nm. An especially important case is that in which the types of parental numbers are divided as seed parents (Nf) and alien fathers, i.e., Nfm and Nm are disregarded, meaning that orchard crops are pollinated entirely by contaminating pollen. For this situation, the status number becomes Ns = 4, assuming that contaminating pollen is related neither to the orchard pollen nor to itself, and contributes equally to the seed parents (IX; Lindgren and Mullin, 1998). The amount of genetic drift in a seed crop where the sex ratio of the parents is skewed is higher than that for a crop where the parental sex ratio is balanced (I). From the sex-ratio standpoint, Ns is the size of an ideal population having a 1:1 sex ratio, which is subject to the same degree of genetic drift as a real orchard population. In other words, the amount of random genetic drift in the orchard population size of N is equal to that in an ideal population of size Ns, where genders are represented equally and mated randomly. It should be noted that inbreeding or genetic drift depends mainly on the numbers of the less-numerous sex (Caballero, 1994). For instance, if an orchard were maintained with an infinitely large number of pollen parents (Nm ) but only one seed parent (Nf = 1), the status effective number would be only four (I and IX). Concerning the number of alleles, allelic diversity is less sensitive to unequal number of sexes. Let’s recall the case of one seed parent (Nf = 1) and 100 pollen parents (Nm = 100). The loss of gene diversity in this case is estimated to be 12.5% relative to the reference, which is the same as an ideal population consisting of four individuals (formula 23). What proportion of allelic diversity will be lost then? This will depend on the number of seed and pollen produced (Allendorf, 1986). A large number of seed crops are produced from seed orchards, so each of the father parents has the opportunity to contribute to the offspring. Thus, there may be a little or no loss of allelic diversity in the progeny. Low frequency alleles may not contribute much to the tree improvement (genetic gain and diversity) in seed orchard programs. 48 5. Over generations Seed orchard programs follow progressive development as each generation produces new parents for the next generation (El-Kassaby and Askew, 1998). In some breeding programs, the seed orchards can function as the sources of bred materials. In such breeding programs, care must be more taken to minimize the problems associated with small effective populations while continuing to achieve an increase in genetic gain. Many factors such as selection criteria of plus trees, arrangement of trees, number of parents, reproductive success and management practices contribute to the realized levels of genetic gain and gene diversity that are achieved in the seed crop (I) and subsequent reforestation stock. An increasing in inbreeding within the population during successive generations of recurrent selection is potentially a major problem in long-term breeding programs (Gea et al., 1997). Inbreeding appears one (if selfing is allowed) or two (if not) generations later than genetic drift caused by the initial selection (Caballero, 1994). Breeding and conservation programs that use seed orchard populations with a low status effective number may lead to a loss of gene diversity in the plantations (VIII; Lindgren et al., 1996). Coancestry of the parental generation becomes inbreeding in the offspring generation if they mate at random (Falconer and Mackay, 1996). In other words, the group coancestry of orchard parents will be the expected group inbreeding of orchard crops following random mating as (III, VIII; Bila, 2000) parents F̂crops [21] In study VIII, an accumulation of group coancestry was monitored over generations. Predictions over five generations showed that group coancestry and inbreeding accumulated quickly at early generation shifts (VIII; Bila, 2000). In the study, the link between the generations was the gamete; hence, the gene pool of the offspring (t) was identical to that of the successful gametes of the parents (t-1). The group coancestry of the gamete gene pool can be described as a function of parent inbreeding (F), fertility variation () and census number (N) as gametes 0.5(1 Ft 1 )Ψ t 1 N t 1 Ψ N 0.5(1 Ft 1 ) 1 t 1 t 1 t 1 N t 1 1 N t 1 [22] Relative status number declined very quickly over generations. The increase in inbreeding and group coancestry was accelerated by fertility variation, and the accumulation was faster and greater when fertility of both genders varied than 49 when maternal fertility was kept constant. Gene diversity was decreased faster when fertility variation was large, but was maintained higher when the orchard population size was reasonably large (VIII). Thus, breeding programs that use small status numbers will lead to the accumulation of inbreeding and group coancestry, and do not provide for a sustainable long-term breeding strategy (Yeh, 2000). Group coancestry increased and gene diversity decreased at an almost constant rate over generations, even if there was high fertility variation. Status numbers dropped remarkably at the first-generation shift (VIII; Bila, 2000), so Ns seems to be less suitable for describing the loss of diversity over generations, especially in long-term breeding populations (Rosvall, 1999). The most appropriate application could be for situations (e.g., seed orchards) concerned with an actual state rather than the rate of change (Lindgren and Mullin, 1998), and in situations where the loss of rare alleles should be emphasized as in small-endangered populations (Ballou and Lacy, 1995). Interpretation of status number Status number is a convenient way of expressing group coancestry in terms of an effective population size. Status number can be seen as the number of genotypes sampled from the reference population of non-inbred, unrelated individuals (Lindgren and Kang, 1997), which has the same group coancestry as the population in question. The status number of an orchard crop shares the following characteristics with the same size of sample from the reference population: the same group coancestry; the same degree of average relatedness; the same probability that genes taken at random from the gamete gene pool are identical by descent; the same expected deviation of gene frequencies from the reference population; the same gene diversity and expected heterozygosity; a similar number of rare alleles; and the same expected inbreeding in the progeny following random mating. Status number can also be interpreted as the number of individuals that are unrelated and non-inbred (II, VII), the number of genotypes having equal fertility (III), or the number of population members having the same sex ratio (I). Status number expresses the accumulated genetic drift from the reference population to which the concepts of inbreeding and coancestry refer (Lindgren et al., 1996). Status number is compared with the traditional concepts of effective population size in Table 5. The variance effective population size (Ne(v)) is the size of an 50 ideal population with the same sampling variance of allelic frequencies per generation (i.e., the same random genetic drift) as the seed orchard (Crow and Kimura, 1970). Ne(v) refers to the variance of change in group coancestry (i.e., status number) from the reference population with all unique alleles (II; Rosvall, 1999). Table 5. Effective numbers studied in seed orchards Type Symbol Definition Reference Status effective number Ns The number of unrelated and non-inbred genotypes, which has the same group coancestry. Sometimes, it can be called the effective number of parents (III). Lindgren et al., 1996; Lindgren & Mullin 1998; Roberds et al., 1991; Skroppa, 1994; Lacy, 1995 Inbreeding effective population size Ne(i) The size of an idealize population that gives the same increase in inbreeding coefficient (in average homozygosity) Wright, 1931; Robertson, 1961; Kimura & Crow, 1963; Pollak, 1977; Harju, 1995 Variance effective population size Ne(v) The size of an idealized population that gives rise to the same expected change (random genetic drift) in gene frequency Crow & Kimura,1970; Crow & Denniston, 1988; Burczyk, 1996; Crossa & Vencovsky, 1997 Eigenvalue effective population size Ne(e) The ideal population size that gives the same expected change in gene frequency and variance in the transition of genes (cf. diversity effective size, random extinction) Haldane, 1939; Crow & Kimura, 1970; Ewens, 1982; Gregorius, 1991; Caballero, 1994 The other conventional effective population size is the inbreeding effective population size (Ne(i)), which is the size of an ideal population that accumulates inbreeding F at the same rate as the orchard population (Kjær and Wellendorf, 1997). If the seed orchard is under Hardy-Weinberg equilibrium (random mating), both Ne(v) and Ne(i) are expected to be identical. While Ne(i) depends primarily on the size of the parental generation, Ne(v) is more dependent on the number of offspring. In contrast to the conventional effective population sizes (Ne(v), Ne(i)), status number (Ns) refers to the accumulated relatedness in the gene 51 pool. Ns is thus an effective number describing at a particular state of development. From Table 5, it is apparent that there are various kinds of effective number, and these can sometimes lead to quite different expressions even though they are usually very similar (Crow and Kimura, 1970; Kjær and Wellendorf, 1997). Wright’s procedure is based on the probability of homozygosity arising from common ancestry, and hence it is called an inbreeding effective number (Caballero, 1994). The amount of allele frequency drift per generation, as measured by its variance, can be used to define a variance effective number (Crow and Denniston, 1988). One can also consider the asymptotic rate of decay of segregating loci, which is determined by the largest non-unit eigenvalue of the transition. Ewens (1982) pointed out that the eignvalue effective number could be estimated by tracing the progress of a quantity such as heterozygosity. The inbreeding effective population size does not make much sense in firstgeneration clonal seed orchards (II). Ne(i) describes how fast a population accumulates inbreeding over generations. If the clones are assumed to be unrelated, inbreeding arises only from selfing. Thus, it is more relevant to describe selfing explicitly, rather than try to base an effective number on it (II; Lindgren and Mullin, 1998). Gregorius (1991) also proposed to use ‘diversity effective size’ that was a derivative of Ne(e). Ns is not informative about selfing, therefore Ne(v) can be a supplement for managing seed orchards and describing their crops. Ns and Ne(v) are also more important than Ne(i) in germplasm collection and regeneration (X; Crossa and Vencovsky, 1997). Gene diversity and heterozygosity Loss of gene diversity by drift in a small population (e.g., seed orchards) results in a loss of allelic variants and lower heterozygosity, while inbreeding due to non-random mating only changes the level of heterozygosity (Rosvall, 1999). Nevertheless, empirical results on variability in natural stands and orchard crops confirm that expected heterozygosities are similar in both populations (Savolainen and Kärkkäinen, 1992). If orchards have a low effective number, some of the important genetic variability may be in the form of rare alleles, e.g., disease resistance. Genetic drift and inbreeding affect both target traits and neutral alleles, while selection (e.g., genetic thinning) will only affect the target and linked genes. Expected gene diversity of seed crops (GD) from first-generation seed orchards can measured relative to the group coancestry of a reference population. GD is a function of group coancestry (Lacy, 1995) and inversely proportional to the status number (II) as 52 GD 1 1 1 2Ns [23] Gene diversity can be defined as the probability that genes are different by descent, and thus the loss of gene diversity compared to an infinite reference population is the accumulation of group coancestry (II; see also Gregorius, 1991). When group coancestry is monitored in seed orchards, one can say how much gene diversity has been lost compared to the reference population, i.e., the wild-forest (Yeh, 2000). Group coancestry also measures gene dispersion (Lindgren and Mullin, 1998). The loss of gene diversity assessed by GD is approximately linear for several generations, reflecting that the amount of additive genetic variance decreases at the same rate as the gene diversity or expected heterozygosity (VIII; Verrier et al., 1991; Savolainen and Kuittinen, 2000). Conversely, the decrease in Ns over generations is non-linear and reflects the sampling process of founder genes, being proportional to the probability that low-frequency genes will be lost (VIII; Lindgren et al., 1996; Rosvall, 1999; Bila, 2000). Gene diversity (GD) is equivalent to expected average heterozygosity (He). The expected average heterozygosity (Het) in orchard crops can thus be demonstrated under drift theory on the same basis as gene diversity (Stoehr and El-Kassaby, 1997; Savolainen and Kuittinen, 2000) as 1 Het 1 2 N e( v ) Het 1 [24] where Ne(v) is the variance effective population size; and Het-1 is the heterozygosity in the parental orchard population (Nei et al., 1975; Lacy, 1995). By definition, the heterozygosity of first-generation orchard parents will be one (Het-1= 1) because all parents are considered to be a sample from the reference population (VII). The expected heterozygosity of orchard crops will thus be the direct function of variance effective population size. If the Ne(v) is larger than 10, the depletion of heterozygosity caused by random drift alone will be very small (Fig. 7). 53 Expected heterzygosity (He ) 1.00 0.75 0.50 0.25 0.00 1 10 20 30 40 50 Variance effective pop. size (Ne (v )) Figure 7. Relationship between expected heterozygosity (He) and variance effective population size (Ne(v)). When Ne(v) is larger than 10, the depletion of heterozygosity caused by genetic drift will be small. Variance effective population size (Ne(v)) is a function of fertility variation ( ) and census number (N) in a seed orchard consisting of unrelated and non-inbred parents, Ne(v) = N/(-1). It describes the change of gene contributions between the orchard parents and their offspring, and the change of status number over generations (I, II; Bila, 2000). When the sibling coefficient is equal to two, = 2 (CV = 100%), Ne(v) will be the same as the orchard census number (N) where parents are unrelated and non-inbred. Concerning gene diversity, the main question is how many parents we have to use in seed orchards. Skroppa (1994) suggested that 20-30 individuals could guarantee a sufficiently high effective population size. If there are too many parents, one can keep much diversity, but selection intensity is low, resulting in little genetic gain (Zobel and Talbert, 1984). It also causes some operational difficulties such as high cost, complicated layout, unfavorable flexibility and so forth. In advanced-generation seed orchards, it is highly recommended to balance high genetic gain with desirable gene diversity. Selfing and inbreeding Selfing is expected in a seed orchard (Rudin and Lindgren, 1977) and will reduce the genetic value of the orchard seeds. The proportion of seed due to selfing is 54 important because of the poor survival and growth of selfed offspring (Franklin, 1970). In clonal seed orchards, selfing can occur by mating among ramets of the same clone as well as self-pollination within individual ramets (II). Frequencies of selfed seedlings vary considerably among clones and years (Squillace and Goddard, 1982). If individual clones have a high level of viable selfed offspring, these clones need to be identified and either rogued from the orchard or treated by pollen management techniques (e.g., SMP) to reduce the amount of selffertilization to acceptable levels (El-Kassaby and Ritland, 1986b). The selfing rate can be estimated from mating probability (Gömöry et al., 2000) or based on isozyme makers (Burczyk, 1991; El-kassaby et al., 1989b; Harju, 1995). In general, selfing rates estimated by isozyme analysis are 2-7% (less than 10%) in clonal seed orchards (Müller-Starck, 1982; Rudin et al., 1986; Adams and Birkes, 1989), but may be higher in particular cases (Rudin and Ekberg, 1982; Lai and Wang, 1997). Selfing tends to be greater in the lower part of crowns (Franklin, 1971b; Rudin and Lindgren, 1977; Shen et al., 1981; Philipson, 1997). Since male strobili occur mainly in the lower and mid-crown, female strobili in the lower crown are more apt to be self-pollinated than those in the upper crown (Squillace and Goddard, 1982), although El-Kassaby et al. (1986) found no significant differences among crown levels and aspects in a Douglas-fir seed orchard. Inbreeding is not desirable in reforestation material (Kang and Namkoong, 1988; Harju, 1995). With proper design in orchard composition and spatial arrangement of parents, inbreeding can be minimized in orchard crops even though inbreeding is substantial in the parent population (Lindgren and El-Kassaby, 1989; Xie and Knowles, 1994). It may be further reduced by SMP and pollen contamination (Rudin and Lindgren, 1977; El-Kassaby, 1995). Inbreeding also affects differently the production of filled seeds, depending on the types of mating (Griffin and Lindgren, 1985). Lindgren and Gregorius (1976) argued that inbreeding might be a tool in a breeding strategy because it increase genetic variance and makes it possible to keep a higher number of unrelated individuals (i.e., higher effective number) in breeding populations. It has been suggested that the genetic cause of embryo collapse producing empty seeds is an inbreeding effect, caused by the action of homozygous lethal genes (Sarvas, 1962). However, the presence of simple recessive embryonic lethals does not satisfactorily explain why the second-generation of selfing appears to yield a much lower percentage of filled seeds than the first-generation of selfing (Andersson et al., 1974; Sniezko, 1984). The study of outcrossing rate in seed orchards is useful for evaluating the rate of inbreeding and its associated effects (El-Kassaby et al., 1989b). In most published reports, high outcrossing rates are observed in orchards (t > 0.9; Friedman and Adams, 1985a; Omi and Adams, 1985; Ritland and El-Kassaby, 55 1985; El-Kassaby et al., 1988). Estimates of outcrossing rate obtained from seed orchards are generally higher, or at least not lower, than those reported for natural stands (Shaw and Allard, 1982; Rudin et al., 1986). It may be due to the population structure, i.e., the physical arrangement of related individuals within a population. In conjunction with the phenomenon of polyembryony, the differential viability of selfed and outcrossed embryos maintains a high effective outcrossing rate in spite of considerable natural self-pollination (Lindgren, 1975). Koski (1973) estimated that, if the level of self-pollination were 20%, there would be less than 1% of trees derived from selfing in regenerated Scots pine stands. Thus, selfing does not seem to be alarming in seed orchards. Under the mixed random mating and selfing model, an effective population size (Ne) reduces to 0.5(1+t)N, where t is the outcrossing rate. Ne is thus always smaller than N in the model, unless the rate of outcrossing is unity. Consequently, if a species exhibits high outcrossing, the effect of inbreeding on the decay of gene diversity is small, relative to that due to genetic drift (Yeh, 2000). Kjær and Welledorf (1997) formulated the inbreeding effective population size, considering inbreeding and selection against self-pollination, as Ne(i)= 1/[(1-t) + w*t(fimi)], where w is the fitness of selfed zygotes compared to outcrossed zygotes (Gömöry et al., 2000). The effect of inbreeding in forest trees is of interest from many points of view. A major question is the evaluation of the hazards involved in selecting related individuals for use in advanced-generation seed orchards. Another aspect is the possibility of using inbreeding as a tool in tree breeding (Andersson et al., 1974; Sniezko, 1984). Estimates of the inbreeding coefficients of orchard crops cannot always be simply estimated, because they are functions of the amount of inbreeding in the current and previous generations. A simple way to determine the degree of expected inbreeding (F) in the seeds from a first-generation seed orchard is (van Buijtenen, 1971) F = (P1 * 0.5) + (P2 * 0.25) + (P3 * 0.125) where P1 = the probability of obtaining seed from selfing P2 = " " " from full-sib crosses P3 = " " " from half-sib crosses From studies II, III, X and XI, the expected inbreeding in orchard crops caused by fertility variation and/or relatedness would be small and could be ignored in first-generation seed orchards. 1. Inbreeding in clonal seed orchards 56 In a clonal seed orchard consisting of unrelated, non-inbred clones, the only factor influencing the degree of inbreeding is the probability P1 of obtaining seed from selfing as P1 = (A1 + A2) C D where A1 is the pollen contribution from the ramet itself; A2 is the pollen contribution from other ramets of the same clone (A2<A1); C is the seed set from selfs (relative value); and D is the proportion of sound seed from selfs (for selfsterile clones, D= 0). The frequency of mutants (e.g., albino, chlorophyll deficient, etc.) appearing in open-pollinated progenies carrying marker genes can give a good estimate of the magnitude of the factor P1 (Squillace and Kraus, 1963) as P1 = Y / X where Y is the percent markers showing in open pollinated seeds; and X is the percent marker showing in the selfed seeds. For instance, 1% albinos are observed in open-pollinated seeds and 16% in selfed seeds, the value of P1 is 0.0625 and F would be 0.031 (Franklin, 1971b; Squillace and Goddard, 1982). From a practical standpoint, orchard managers need not be very concerned about the yield of selfed seedlings from seed orchards. Admittedly, since selfs are usually inferior in growth rates and less vigorous than cross-pollinated seedlings, they are likely to be culled in the nursery or often will be suppressed and die when out-planted. 2. Inbreeding in seedling seed orchards The situation in seedling seed orchards is much more complicated because selfing, crossing among full sibs or crossing among half-sib may all occur. Thus, P1 cannot be modified readily by the design of the orchard, but P 2 and P3 can be modified if separation between half-sibs by one position is considered adequate, which is still not easy. Inbreeding reduces seed and wood production, although a higher genetic gain due to a more intense and unrestricted selection may be obtained if related individuals are used in the same seed orchard (Lindgren and Gregorius, 1976). Williams and Savolainen (1996) advocated using experimental inbred populations for direct utilization of inbreeding as a breeding method. Matheson et al. (1995) reported that it was better to allow some proportion of related mating in the order of parent-offspring pairs, half-sib and full-sib families. In advanced-generation seed 57 orchards, therefore, it is advisable to consider the possibility of using a limited amount of related individuals in the same seed orchard (VII), based on detailed calculations of the expected gain from different alternatives. It could be a kind of linear deployment (Lindgren and Matheson, 1986) or step-wise arrangement (Hodge and White, 1993). In general, it is better to include more genetic entries with fewer individuals per entry rather than vise versa for less inbreeding in seed crops. It may also appear better to use more ramets of the best relative and thus fewer entries for high gain than to include several relatives if the inbreeding is acceptable. 3. Inbreeding depression Inbreeding depression can be defined as the reduction in seed production caused by inbreeding compared with the seed production from outcrossing. It can be caused by heterozygote superiority at specific loci (over-dominance hypothesis) or arise from recessive or partly recessive deleterious genes (dominance hypothesis) (Crow, 1952; Charlesworth and Charlesworth, 1987; see also review by Williams and Savolainen, 1996). The latter one is suggested as the main contributor to inbreeding depression, which is maintained by mutation-selection balance. Inbreeding depression expressed during seed development is especially severe in conifers (Sorensen, 1969; Koski, 1971, 1973; Lindgren, 1975), although there may be strong selection against deleterious genes or recessive mutations due to rather frequent self-fertilization. Griffin and Lindgren (1985) suggested that gain estimates should be adjusted downward at least in proportion to the expectation of sib-mating (i.e., inbreeding depression), if breeders were to include multiple selections from full- or half-sib families in the seed orchards. Surprisingly, there was little inbreeding depression in germination rate and percent mortality through two generations of selfing in loblolly pine (Sneizko, 1984). Inbreeding depression on height growth in the nursery depends also on growth conditions in Norway spruce (Andersson et al., 1974). On the other hand, inbreeding depression for survival and growth vigor was consistent through three generations of selfing in silver birch (Wang et al., 1999). Inbreeding depression on height growth of S1 progeny has a large range from zero to almost 50%, averaging 22%, for 20 conifer species (see Table 8.2 in Sedgley and Griffin, 1989). It seems that less inbreeding depression is found among families produced from parents with high GCA for growth (Mullin et al., 1978). When the population size is kept constant, and each parental genotype is equally represented, serious inbreeding and inbreeding depression can be avoided for many cycles of selection (Barnes, 1995). Inbreeding depression does tend to be linear with the respect to the coefficient of inbreeding (F) if there is no epistatic interaction among loci (Sniezko, 1984). As 58 inbreeding accumulates, reproductive capacity will be deteriorate. Due to the both factors, it soon becomes impossible to avoid the loss of some individuals or of some lines. The survivors are then a selected group to which the theoretical expectations no longer apply. The precise measurement of the rate of inbreeding depression can thus generally be made only over the early stages, before the inbreeding coefficient reaches high levels (Falconer and Mackay, 1996). Inbreeding depression results in reduced yield of viable seeds, poor germination, segregation of deformed or chlorophyll deficient seedlings, and varying degrees of reduced growth (Franklin, 1970). As discussed above, however, the outcrossing rate in most conifer species is very high, selfed embryos are killed by lethal alleles, selfed seedlings are often out-competed in the nursery, and very seldom do they remain in the mature stand. Thus, selfing in plantations derived from orchard crops has probably more or less negligible effects. 59 Concluding remarks Genetic gain and gene diversity of orchard crops were evaluated in seed orchard populations. High genetic gain could be obtained using orchard management alternatives (e.g., selective cone harvest, genetic thinning), while reasonable gene diversity (measured by status number) was maintained. Effective management of forest genetic resources is a key element in future forestry. In the future, seed orchards must balance genetic gain and gene diversity, in order to ensure high genetic quality of orchard crops. There was a large variation in ramet number and flower production among parents in seed orchards, although the sibling coefficient was generally in the order of = 2 (CV = 100%). Reproductive characteristics were under genetic control and positively correlated with years. Fertility variation will accelerate the accumulation of group coancestry and inbreeding, and hence reduce the expected genetic gain and gene diversity of seed orchard crops. Relative status number (Nr) was estimated to be 0.74 due to the ramet variation. By increasing seed quality and quantity in seed orchards rather than by establishing larger area, seed orchard genetics in combination with proper silvicultural management will provide better opportunity for the use of seed orchards for breeding and conservation purposes. If the status number (Ns) of an orchard crop was larger than 10, the depletion of gene diversity in the following generation, due to genetic drift and fertility variation, would be small. On the other hand, there would be lower expected genetic gain and a potential inbreeding problem in seed orchards or breeding populations with the status number of less than 10. A large number of parents are generally used in first-generation seed orchards; thus, genetic loss and erosion due to genetic drift do not seem to be alarming during the process of domestication, because of the large number of orchard parents. Gene diversity should, however, be monitored and controlled to maintain as much diversity as possible in future-generation seed orchards. Seed orchards are the link between breeding programs and reforestation through the delivery of consistent, abundant yields of genetically improved seed. Seed orchard crops represent the mechanism by which new forests are created and genetic variation is transmitted from one generation to another. Status number (Ns) is a useful tool for monitoring gene diversity of orchard crops. Status number will also bring better information for seed certification in future seed orchards. To describe the effect of random genetic drift, Ns and Ne(v) seem to be more informative than Ne(i) in seed orchard populations. When the desirable level of effective number is maintained, seed orchards can produce orchard crops with high genetic gain and gene diversity. An understanding of reproductive processes (e.g., random mating, phenological synchrony, parental balance) and the impact of management practices (e.g., equal 60 seed harvest, genetic thinning, SMP, overhead cooling, and mixing seeds from different years) is essential for maximizing genetic gain obtained from seed orchard programs and for maintaining the gene diversity required to meet environmental contingencies. Management options such as selective harvesting, partial silvicultural thinning, genetic roguing, flower induction and SMP are available for orchard managers to promote better reproductive balance in seed orchards. Orchard managers should consider options that are cheaper and more practical. Much closer attention also needs to be focused on reproductive balancing when seed orchards are rogued in order to raise the overall average breeding value of parents represented and thus maintain durable gene diversity of reforestation stock. Realized genetic gain and gene diversity of orchard crops usually deviate from the expectation for an ideal case. For the successful functioning of seed orchards, orchard managers, specialists and researchers should diligently observe, assiduously collect and consistently study all essential information on parental behavior, compatibility, combining ability and gene flow, and then translate this information into practical terms so that it may be employed in future seed orchards. Genetic gain and gene diversity will vary considerably from orchard to orchard, and from year to year. Cost and time for establishment and management will also vary. Gene diversity can have importance for both the long-term stability and the short-term productivity of forest plantations. Hence, there is need for information on seed orchard genetics in the near future. Furthermore, tree improvement through seed orchards is probably one of the most cost-effective alternatives available to forest managers to improve forest productivity. Modern seed orchard research is the science, business and art of managing and conserving genetic resources for continuing economic, social and environmental benefit. It involves the balanced utilization and optimum management of forest resources for maximum yields of improved seed from seed orchards. Recent orchard study also draws upon the knowledge and experience from many disciplines and other professions, and plays a wide role in the development and implementation of new techniques. Orchard seeds yield good forests and give additional protection because they contain the offspring of carefully selected superior parents. Plantations from seed orchard crops can also provide more protection against disease or insect catastrophe, adapt well to varying site and soil conditions, and enhance biological diversity. 61 Future perspectives related to this thesis Relatedness among candidates for seed orchards will increase in advancedgeneration breeding programs, and relatives will likely be used in future seed orchards. The effect of mating between relatives (among ramets of related clones or among related trees) needs to be better evaluated, so the breeder knows better in advanced-generation seed orchards whether relatives with high genetic values can be effectively used or not. Orchard managers will want to get high genetic gain rather than high diversity even if some inbreeding depression may result. However, the question of how much gain and diversity are sacrificed when relatives are allowed to mate in seed orchards is still not fully analyzed. The outcome of a seed orchard is also dependant on selfing. Selfing is an important consideration, particularly when the need for a higher effective number of clones is considered. Thus, the effect of selfing and its relationship to effective number in seed orchards ought to be better studied. In previous studies, which mainly dealt with clonal seed orchards, the assumption was normally made that orchard parents were unrelated. This may have been a reasonable attitude in the past and even for the present, for most attention has been on clonal seed orchards. Still, there will be relatives in seedling seed orchards (breeding seedling orchards) and in advanced-generation seed orchards. Formulae for genetic gain and gene diversity in future-generation seed orchards with complicated pedigree should be developed. More practical variables, such as inbreeding, inbreeding depression and seed production, should also be considered for optimizing and balancing genetic gain and gene diversity of orchard crops that will give maximum orchard benefit. For contaminating pollen, it was assumed in most studies that the contribution of the foreign pollen was uniform and equal for all orchard parents, which may not be true. Flower phenology and synchrony between the pollen producers and female flowers were not considered, and observations that allow for generalizations were difficult to obtain. By considering phenological aspects, the impact of pollen viability, pollen density, pollen dispersal, seed size, germination parameters, seed viability and seedling production on the genetic gain and gene diversity of orchard crops can be better analyzed. The study showed that controlling female fertility (i.e., equal seed harvest or mixing seed equally among parents) increased gene diversity of seed orchard crops. However, if an amount of seed production is an urgent matter in a seed orchard, one could elaborate on the point with balancing on the male side rather than the female side by removing ramets of a clone or by controlling family sizes. This could be done without loss of seed if there is sufficient pollen, while it increases gene diversity. The problem is that the female-male correlations apparently vary among years. From theoretical and practical points of view, it 62 could be interesting to investigate the controlling male fertility and its effect on genetic gain and diversity of orchard crops. Linear deployment, using orchard parents in proportions linearly related to their breeding values, gives an optimal compromise between genetic gain and gene diversity of crops from unrelated, non-inbred clones. More appropriate orchard deployment principles will be needed in advanced-generation seed orchards where the parents will be related. Algorithms and software should also be developed for designing of future seed orchards and for cheaper forest improvement options including seed stands and seedling seed orchards. Computer-based tools (by MS® Excel) in seed orchard management and some useful programs in breeding theory can be found at the web-site managed by Prof. Dag Lindgren; http://www.genfys.slu.se/staff/dagl/Breed_Home_Page/ 63 References Adams, W.T. and Birkes, D.S. 1989. Mating patterns in seed orchards. In: Proc. 20th SFTIC conf. Charleston, South Carolina. p.75-86. Adams, G.W. and Kunze, H.A. 1996. Clonal variation in cone and seed production in black and white spruce seed orchards and management implications. For. Chron. 72: 475-480. Adams, W.T., Griffin, A.R. and Moran, G.F. 1992. Using paternity analysis to measure effective pollen dispersal in plant populations. American Naturalist 140: 762-780. Adams, W.T., Hipkins, V.D., Burczyk, J. and Randall, W.K. 1997. Pollen contamination trends in a maturing Douglas-fir seed orchard. Can. J. For. Res. 27: 131-134. Ahlgrn, C.E. 1972. Some effects of inter- and intraspecific grafting on growth and flowering of some five-needle pines. Silvae Genet. 21: 122-126. Allendorf, F.W. 1986. Genetic drift and the loss of alleles versus heterozygosity. Zoo Biology, 5: 181-190. Allison, T. 1990. Pollen production and plant density affect pollination and seed production in Taxus canadensis. Ecology 71: 516-522. Andersson, E.W. 1999. Gain and diversity in multi-generation breeding programs. Ph.D. thesis. Swedish University of Agricultural Sciences, Umeå, Sweden. Acta Universitatis Agriculturae Sueciae, Silvestria 95, 1999. Andersson, E. 1955. Pollen dispersal and the isolation by distance of seed orchards. Norrlands SkogsvFörb. Tidsskr., Stockh. 1: 35-100. Andersson, E., Jansson, R. and Lindgren. D. 1974. Some results from second generation crossings involving inbreeding in Norway spruce (Picea abies). Silvae Genet. 23: 34-43. Askew, G.R. 1988. Estimation of gamete pool composition in clonal seed orchards. Silvae Genet. 37: 227-232. Askew, G.R. and Burrows, P.M. 1983. Minimum coancestry selection I. A Pinus taeda population and its simulation. Silvae Genet. 32: 125-131. Ballou, J.D. and Lacy, R.C. 1995. Identifying genetically important individuals for management of genetic variation in pedigreed populations. In: Population management for survival and recovery: Analytical methods and strategies in small population conservation. Columbia Univ. Press. NY. p.76-111. Barnes, R.D. 1995. The breeding seedling orchard in the mutiple population breeding strategy. Silvae Genet. 44: 81-88. Bergman, F. and Ruetz, W. 1991. Isozyme genetic variation and heterozygosity in random tree samples and selected orchard clones from the same Norway spruce populations. For. Ecol. Manage. 46: 39-47. Bila, A.D. 2000. Fertility variation and its effects on gene diversity in forest tree populations. Ph.D. thesis. Swedish University of Agricultural Sciences, Umeå, Sweden. Acta Universitatis Agriculturae Sueciae, Silvestria 166, 2000. Bishir, J. and Roberds, H. 1999. On number of clones needed for managing risks in clonal forestry. For. Genet. 6: 149-155. 64 Bjornstad, A. 1981. Photoperiodical after-effect of parent plant environment in Norway spruce (Picea abies (L.) Karst) seedlings. Medd. Nor. Inst. Skogforsk 36: 1-30. Bridgwater, F.E. and Trew, I.F. 1981. Supplemental mass pollination. In: Pollen management handbook 587. Ed) Dr. Franklin, E.C. Washington, D.C. p.15-19. Bridgwater, F.E., Bramlett, D.L., Byram, T.D. and Lowe, W.J. 1998. Controlled mass pollination in loblolly pine to increase genetic gains. For. Chron. 74: 185-189. Brisbane, J.R. and Gibson, J.P. 1995. Balancing selection response and rate of inbreeding by including genetic relationships in selection decisions. Theor. Appl. Genet. 91: 421-431. Brown, I. 1971. Flowering and seed production in grafted clones of Scots pine. Silvae Genet. 20: 121-132. Buckland, D.C. and Kuijt, J. 1957. Unexplained brooming of Douglas-fir and other conifers in British Columbia and Alberta. For. Sci. 3: 236-242. Burczyk, J. 1991. The mating system in a Scots pine clonal seed orchard in Poland. Ann. Sci. For. 48: 443-451. Burczyk, J. 1996. Variance effective population size base on multilocus gamete frequencies in coniferous populations: an example of a Scots pine clonal seed orchard. Heredity 77: 74-82. Burczyk, J. and Prat, D. 1997. Male reproductive success in Pseudotsuga menziesii (Mirb.) Franco: the effects of spatial structure and flowering characteristics. Heredity 79: 638-647. Byram, T.D., Bridgwater, F.E., Gooding, G.D. and Lowe, W.J. 1998. 46th progress report of the cooperative forest tree improvement program. Texas Forest Service, 23p. Byram, T.D., Lowe, W.J. and McGriff, J.A. 1986. Clonal and annual variation in cone production in loblolly pine seed orchards. For. Sci. 32: 1067-1073. Caballero, A. 1994. Developments in the prediction of effective population size. Heredity 73: 657-679 Cameron, R.J. and Thomson, G.V. 1971. An instance of clonal incompatibility in grafted Pinus radiata seedlings. Silvae Genet. 20: 195-199. Caron, G.E. 1987. Pollen monitoring in two black spruce seedling seed orchards. Fraser Inc., Edmonston, N.B. Chaisurisri, K. and El-Kassaby, Y.A. 1993. Estimation of clonal contribution to cone and seed crops in a Stika spruce seed orchard. Ann. Sci. For. 50: 461-467. Chaisurisri, K. and El-Kassaby, Y.A. 1994. Genetic diversity in a seed production population vs natural population of Sitka spruce. Biodiv. Conserv. 3: 512-523. Chalupka, W. 1980. Regulation of flowering in Scots pine (Pinus sylvestris L.) grafts by gibberellins. Silvae Genet. 29: 118-121. Chalupka, W. 1998. Pollen formed under pollution affects some quantitative characters of Scots pine (Pinus sylvestrys L.) seeds. For. Genet. 5: 133-136. Charlesworth, D. and Charlesworth, B. 1987. Inbreeding depression and its evolutionary consequences. Ann. Rev. Ecol. Syst. 18: 237-268. Cheliak, W.M., Murray, G. and Pitel, J.A. 1988. Genetic effects of phenotypic selection in white spruce. For. Ecol. Manage. 24: 139-149. Clare, L.R. 1982. Assessment of pollen contamination in Pacific Forest Products’ high elevation seed orchard. BSF. thesis, Univ. of British Columbia, Vancouver. 65 Climent, J.M., Prada, M.A. and Pardos, J.A. 1997. Increase of flowering in Pinus nigra Arn subsp salzmanni (Dunal) Franco by means of heteroplastic grafts. Ann. Sci. For. 54: 145-153. Cockerham, C. 1967. Group inbreeding and coancestry. Genetics 56: 89-104. Crossa, J. and Vencovsky, R. 1997. Variance effective population size for two-stage sampling of monoecious species. Crop Sci. 37: 14-26. Crow, J.F. 1952. Dominance and overdominance. In: Heterosis, ed) Gowen, J.W. Iowa State College Press, Ames, p.282-297. Crow, J.F. and Denniston, C. 1988. Inbreeding and variance effective population numbers. Evolution 42: 482-495. Crow, J.F. and Kimura, M. 1970. An introduction to population genetics theory. Harper and Row Publishers, London, 591pp. Danbury, D.J. 1971. Economic implications of selection for seed production in radiata pine seed orchards. Aust. For. Res. 5: 37-44. Darwin, C. 1877. The differential forms of flowers on plants on the same species. John Murray, London. Denison, N.P. 1973. A review of aspects of tree breeding in the Republic of South Africa. In: Selection and breeding to improve some tropical conifers. Eds) Drs. Burley, J. and Nikles, D.G. Oxford. Comm. For. Inst. 2: 277-284. Devlin, B. and Ellstrand, N.C. 1990. Male and female fertility variation in wild radish, a hermaphrodite. Am. Nat. 136: 87-107. Di-Giovanni, F. and Kevan, P.G. 1991. Factors affecting pollen dynamics and its importance to pollen contamination: a review. Can. J. For. Res. 21: 1155-1170. Dormling, I. and Johnsen, O. 1992. Effects of the parental environment on full-sib families of Pinus sylvestris. Can. J. For. Res. 22: 88-100. Duffield, J.W. and Wheat, J.G. 1963. Dwarf seedling from broomed Douglas-fir. Silvae Genet. 12: 129-133. Duzan, H.W. and Williams, C.G. 1988. Matching loblolly pine families to regeneration sites. Southern J. of Applied Forestry 12: 166-169. Dyson, W.G. 1975. A note on dwarfing of Pinus patula grafts. Silvae Genet. 24: 6061. Edwards, D.G.W. and El-Kassaby, Y.A. 1996. The biology and management of coniferous forest seeds: genetic perspectives. For. Chron. 72: 481-484. El-Kassaby, Y.A. 1995. Evaluation of tree-improvement delivery system: factors affecting genetic potential. Tree Physiology 15: 545-550. El-Kassaby, Y.A. and Askew, G.R. 1991. The relation between reproductive phenology and reproductive output in determining the gametic pool profile in a Douglas-fir seed orchard. For. Sci. 37: 827-835. El-Kassaby, Y.A. and Askew, G.R. 1998. Seed orchards and their genetics. In: Forest Genetics and Tree Breeding, Chap. 6: p.103-111. A.K. Mandal and G.L. Gibson (eds.). CBS Publishers and Distributors. Daryaganj, New Delhi. El-Kassaby, Y.A. and Cook, C. 1994. Female reproductive energy and reproductive success in a Douglas-fir seed orchard and its impact on genetic diversity. Silvae Genet. 43: 243-246. El-Kassaby, Y.A. and Davidson, 1990. Impact of crop management practices on the seed crop genetic quality in a Douglas-fir seed orchard. Silvae Genet. 39: 230-237. 66 El-Kassaby, Y.A. and Namkoong, G. 1995. Genetic diversity of forest tree plantations: consequences of domestication. In: Consequences of changes in biodiversity. IUFRO World Congress, Tampere, Finland, Vol 2. p.218-228. El-Kassaby, Y.A. and Reynolds, S. 1990. Reproductive phenology, parental balance, and supplemental mass pollination in a Sitka-spruce seed orchard. For. Ecol. Manage. 31: 45-54. El-Kassaby, Y.A. and Ritland, K. 1986a. Low levels of pollen contamination in a Douglas-fir seed orchard as detected by allozyme makers. Silvae Genet. 35: 224229. El-Kassaby, Y.A. and Ritland, K. 1986b. The relation of outcrossing and contamination to reproductive phenology and supplemental mass pollination in a Douglas-fir seed orchard. Silvae Genet. 35: 240-244. El-Kassaby, Y.A. and Thomson, A.J. 1996. Parental rank changes associated with seed biology and nursery practices in Douglas-fir. For. Sci. 42: 228-235. El-Kassaby, Y.A., Fashler, A.M.K. and Sziklai, O. 1984. Reproductive phenology and its impact on genetically improved seed production in a Douglas-fir seed orchard. Silvae Genet. 33: 120-125. El-Kassaby, Y.A., Parkinson, J. and Devitt, W.J.B. 1986. The effect of crown segment on the mating system in a Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) seed orchard. Silvae Genet. 35: 149-155. El-Kassaby, Y.A., Ritland, K. Fashler, A.M.K and Devitt, W. 1988. The effect of reproductive phenology upon the mating structure of a Douglas-fir seed orchard. Silvae Genet. 37: 76-82. El-Kassaby, Y.A., Fashler, A.M.K. and Crown, M. 1989a. Variation in fruitfulness in a Douglas-fir seed orchard and its effect on crop-management decisions. Silvae Genet. 38: 113-121. El-Kassaby, Y.A., Rudin, D. and Yazdani, R. 1989b. Levels of outcrossing and contamination in two Pinus sylvestris L. seed orchards in Northern Sweden. Scand. J. For. Res. 4: 41-49. El-Kassaby, Y.A., Edwards, D.G. and Cook, C. 1990. Impact of crop management practices on seed yield in a Douglas-fir seed orchard. Silvae Genet. 39: 226-230. Eriksson, U. 1996. Enhancing production of high-quality seed in Swedish conifer breeding. Ph.D. thesis. Swedish University of Agricultural Sciences, Uppsala, Sweden. Eriksson, G., Jonsson, A. and Lindgren, D. 1973. Flowering in a clone trial of Picea abies Karst. Stud. For. Suec. 110. 45pp. Ewens, W.J. 1982. On the concept of the effective population size. Theoretical Population Biology 21: 373-378. Falconer, D.S. and Mackay, T.F.C. 1996. Introduction to quantitative genetics. 4th edition. Longman, Malaysia. 464pp. Fashler, A.M.K. and Devitt, W.J.B. 1980. A practical solution to Douglas-fir seed orchard pollen contamination. For. Chron. 56: 237-241. Fashler, A.M.K. and El-Kassaby, Y.A. 1987. The effect of water spray cooling treatment on reproductive phenology in a Douglas-fir seed orchard. Silvae Genet. 36: 245-249. Feilberg, J. and Soegaard, B. 1975. ’Historical review of seed orchards’. Forestry Comm. Bull. No.54. Her Majesty’s stationary office, London. 67 Frankel, O.H. and Soulé, M.E. 1981. Population genetics and conservation. In: Conservation and evolution. Cambridge University Press.Cambridge,UK. p.31-77. Franklin, E.C. 1970. Survey of mutant forms and inbreeding depression in species of the family Pinaceae. USDA For. Serv. Res. Paper SE-61. USDA. Ashville, NC. Franklin, E.L. 1971a. Pollen management in southern seed orchard. In: Proc. 11th SFTIC, Atlanta, Georgia, USA. p.218-223. Franklin, E.C. 1971b. Estimation of frequency of natural selfing and of inbreeding coefficients in loblolly pine. Silvae Genet. 20: 194-195. Friedman, S.T. and Adams, W.T. 1981. Genetic efficiency in loblolly pine seed orchards. In: Proc. 16th SFTIC conf. Blacksburg, VA. p.213-224. Friedman, S.T. and Adams, W.T. 1985a. Levels of outcrossing in two loblolly pine seed orchards. Silvae Genet. 34: 157-162. Friedman, S.T. and Adams, W.T. 1985b. Estimation of gene flow into two seed orchards of loblolly pine (Pinus taeda L.). Theor. Appl. Genet. 69: 609-615. Fries, A. 1994. Development of flowering and the effect of pruning in a clonal seed orchard of lodgepole pine. Can. J. For. Res. 24: 71-76. Gardner V.R., Bradford, F.C. and Hooker, H.D. 1922. Fundamentals of fruit production. MacGraw-Hill, New York. p.551-579. Gea, L.D., Lindgren, D., Shelbourne, C.J.A. and Mullin, T. 1997. Complementing inbreeding coefficient information with status number: implications for structuring breeding populations. NZ J. For. Sci. 27: 255-271. Goddard, R.E. 1964. The frequency and abundance of flowering in a young slash pine orchard. Silvae Genet. 13: 184-186. Gömöry, D. and Paule, L. 1992. Inferences on mating system and genetic composition of a seed orchard crop in the European larch (Larix decidua Mill.). J. Genet. & Breed. 46: 309-314. Gömöry, D., Bruchanik, R. and Paule, L. 2000. Effective population number estimation of three Scots pine (Pinus sylvestris L.) seed orchards based on an integrated assessment of flowering, floral phenology, and seed orchard design. For. Genet. 7: 65-75. Gregorius, H.R. 1989. Characterization and analysis of mating system. Ekopan Verlag, Germany, 158pp. Gregorius, H.R. 1991. On the concept of effective number. Theor. Pop. Biol. 40: 269283. Griffin, A.R. 1982. Clonal variation in Radiata pine seed orchards. I. Some flowering, cone and seed production traits. Aust. For. Res. 12: 295-302. Griffin, A.R. and Lindgren, D. 1985. Effect of inbreeding on production of filled seed in Pinus radiata experimental results and a model of gene action. Theor. Appl. Genet. 71: 334-343. Griffin, A.R., Wong, C.Y., Wickneswari, R. and Chia, E. 1992. Mass production of hybrid seed of Acacia mangium x Acacia auriculiformis in biclonal seed orchards. In: Breeding technologies for tropical acacias. ACIAR-Proceedings, No. 37. Proc. of an international workshop, Tawau, Sabah, Malaysia, 1-4 July 1991. p.70-75. Hadders, G. 1972. Kontroll av inkorsningen i en tallplantage. Foren. Skogstradsforadlings Arsb. Uppsala. 1972. Haldane, J.B.S. 1939. The equilibrium between mutation and random extinction. Ann. Eugen. 9: 400-405. 68 Harju, A. 1995. Genetic functioning of Scots pine seed orchards. Ph.D. thesis. University of Oulu, Finland. Acta Universitatis Ouluensis 271. Harju, A.M. and Nikkanen, T. 1996. Reproductive success of orchard and nonorchard pollens during different stages of pollen shedding in a Scots pine seed orchard. Can. J. For. Res. 26: 1096-1102. Hartmann, H.T., Kester, D.E., Davies, F.T. and Geneve, R.L. 1997. Techniques of seed production and handling. In: Plant propagation – principles and practices. Sixth edition. Prentice Hall International, Inc. p.147-172. Häcker, M. and Bergmann, F. 1991. The proportion of hybrids in seed from a seed orchard composed of two larch species (L. europaea and L. leptolepis). Ann. Sci. For. 48: 631-640. Hellebergshaugem, F. 1970. Seed orchards as seed sources in forestry. Norsk Skogbr. 16(20): 420-422. Ho, R.H. and Schooley, H.O. 1995. A review of tree crown management in conifers orchards. For. Chron. 71: 311-316. Hodge, G.R. and White, T.L. 1993. Advanced-generation wind-pollination seed orchard design. New For. 7: 213-236. Jones, N. and Burley. J. 1973. Seed certification, provenance nomenclature and genetic history in forestry. Silvae Genet. 22: 53-58. Jonsson, A., Ekberg, I. and Eriksson, G. 1976. Flowering in a seed orchard of Pinus sylvestris L. Stud. For. Suec. 165. 38pp. Kang, H. and Namkoong, G. 1988. Inbreeding effective population size under some artificial selection schemes. 1. Linear distribution of breeding values. Theor. Appl. Genet. 75: 333-339. Kärkkäinen, K. 1994. Deleterious mutations and sexual allocation in the evolution of reproduction of Scots pine. Ph.D. thesis. Oulu University, Finland. Kellison, R.C. 1971 Seed orchard management. 11th congress on SFTIC, Atlanta, Ga., p.160-172. Kimura, M. and Crow, J.F. 1963. The measurement of effective population number. Evolution 17: 279-288. Kjær, E.D. 1996. Estimation of effective population number in a Picea abies (Karst.) seed orchard based on flower assessment. Scand. J. For. Res. 11: 111-121. Kjær, E.D. and Wellendorf, H. 1997. Variation in flowering and reproductive success in a Danish Picea abies (Karst.) seed orchard. For. Genet. 4: 181-188. Knowles, P. 1985. Comparison of isozyme variation among natural stands and plantations: jack pine and black spruce. Can. J. For. Res. 15: 902-908. Kormut'ák, A. and Lindgren, D. 1996. Mating system and empty seeds in silver fir (Abies alba Mill.). For. Genet. 3: 231-235. Koski, V. 1970. A study of pollen dispersal as a mechanism of gene flow in conifers. Comm. Inst. For. Fenn. 70. Helsinki, 1970. 78pp. Koski, V. 1971. Embryonic lethals of Picea abies and Pinus sylvestris. Commun. Inst. For. Fenn. 75.3. Helsinki, 1973. 30pp. Koski, V. 1973. On self-pollination, genetic load, and subsequent inbreeding in some conifers. Commun. Inst. For. Fenn. 78.10. 40pp. Koski, V. 1980a. On the variation of flowering and seed crop in nature stands of Pinus sylvestris L. Silva Fenn. 14: 71-75. 69 Koski, V. 1980b. Minimum requirements for seed orchards of Scots pine in Finland. Silva Fenn. 14: 136-149. Kossuth, S.V., McCall, E. and Ledbetter, J. 1988. Clone certification by use of cortical monoterpenes as biochemical markers. Silvae Genet. 37: 73-76. Krebs, C.J. 1985. The experimental analysis of distribution and abundance. 3rd edition. Harper and Row, NY. Lacy, R.C. 1995. Clarification of genetic terms and their use in the management of captive populations. Zoo Biology 14: 565-578. LaDeau, S.L. and Clark, J.S. 2001. Rising CO2 levels and the fecundity of forest trees. Science 292: 95-98. Lai, H.-L. and Wang, Z.-R. 1997. Advance in research on mating system of forest populations. World Forestry Research 10: 10-15. Lawlor, W. 1991. In: Impact of global climatic changes on photosynthesis and plant productivity. Eds) Y.P. Abrol et al., Oxford & IBB Publishing, New Delhi, India. p.431. Li, B., McKeand, S and Weir, R. 1999. Tree improvement and sustainable forestry impact of two cycles of loblolly pine breeding in the USA. For. Genet. 6: 229-234. Lindgren, D. 1975. The relationship between self-fertilization, empty seeds and seeds originating selfing as a consequence of polyembryony. Stud. For. Suec. 126. 24pp. Lindgren, D. and El-Kassaby, Y.A. 1989. Genetic consequences of combining selective cone harvesting and genetic thinning in clonal seed orchards. Silvae Genet. 38: 65-70. Lindgren, D. and Gregorius, H.R. 1976. Inbreeding and coancestry. In: Proc. IUFRO working parties on population and ecological genetics, breeding theory, biochemical genetics and progeny testing. 1976. Bordeaux, France. p.49-72. Lindgren, D. and Kang, K.S. 1997. Status number - a useful tool for tree breeding. Res. Rep., For. Gen. Res. Inst. Korea. 33: 154-165. Lindgren, D. and Matheson, A.C. 1986. An algorithm for increasing the genetic quality of seed from seed orchards by using the better clones in higher proportions. Silvae Genet. 35: 173-177. Lindgren, D. and Mullin, T.J. 1997. Balancing gain and relatedness in selection. Silvae Genet. 46: 124-129. Lindgren, D. and Mullin, T.J. 1998. Relatedness and status number in seed orchard crops. Can. J. For. Res. 28: 276-283. Lindgren, D. and Wei, R.-P. 1994. Effects of maternal environment on mortality and growth in young Pinus sylvestris in field trials. Tree Physiology 14: 323-327. Lindgren, D., Gea, L. and Jefferson, P. 1996. Loss of genetic diversity monitored by status number. Silvae Genet. 45: 52-59. Lindgren, D., Gea, L. and Jefferson, P. 1997. Status number for measuring genetic diversity. For. Genet. 4: 69-76. Matheson, A.C., Eldridge, K.G., Brown, A.G. and Spencer, D.J. 1986. Wood volume gains from first-generation radiata pine seed orchards. CSIRO Div. For. Res. User Ser No. 4, 13pp. Matheson, A.C., White, T.L. and Powell, G.R. 1995. Effects of inbreeding on growth, stem and rust resistance in Pinus elliottii. Silvae Genet. 44: 37-46. Matthews, J.D. 1963. Factors affecting the production of seed by forest trees. Forestry abstracts 24 (1), 13pp. 70 Matziris, D. 1994. Genetic variation in the phenology of flowering in Black pine. Silvae Genet. 43: 321-328. Mikola, J. 1993. Provenance and individual variation in climatic hardness of Scots pine in northern Finland. In: Forest development in cold climates. Ed) Alden, J. Plenum press, New York, p.333-342. Muhs, H.J. 1986. A synopsis of some international and national rules for forest reproductive material moving into trade-OECD scheme, EEC directives and German law of forest reproductive material. Proc. IUFRO J. Mtg. of WP S2.04-05 and S2.03-14. Grosshansdorf, Germany. 25-28 June 1985. p.25-37. Mullin, L.J., Barnes, R.D. and Prevost, M.J. 1978. A review of the Southern Pines in Rhodesia. Rhod. Bull. For. Res. No. 7. Rhodesia Forestry Commission. 328pp. Müller-Stark, G. 1982. Tracing external pollen contribution to the offspring of Scots pine seed orchard. In: Proc. IUFRO Mtg. on breeding strategies including multiclonal varieties. Lower Saxony FRI, Dep. of For.Tree Breeding, Escherode, Germany. p.176. Nagasaka, K. and Szmidt, A.E. 1985. Multilocus analysis of external pollen contamination of a Scots pine (Pinus sylvestris L.) seed orchard. In: Population genetics in forestry. Ed) Dr. Gregorius H.R. Lecture notes in biomathematics 60: 134-138. Namkoong, G., Snyder, E.B. and Stonecypher, R.W. 1966. Heritability and gain concepts for evaluating breeding systems such as seedling orchards. Silvae Genet. 15: 76-84. Nei, M. Maruyama, T. and Chakraborty, R. 1975. The bottleneck effect and genetic variability in populations. Evolution 29: 1-10. Nikkanen, T. and Ruotsalainen, S. 2000. Variation in flowering abundance and its impact on the genetic diversity of the seed crop in a Norway spruce seed orchard. Silva Fenn. 34: 205-222. Nikkanen, T. and Velling, P. 1987. Correlations between flowering and some vegetative characteristics of grafts of Pinus sylvestris. For.Ecol.Manage.19: 35-40. OECD. 1974. Establishing an OECD scheme for the control of forest reproductive material moving in international trade. C(74)29(final). 23p. Olsson, T., Lindgren, D. and Ericsson, T. 2000. A comparison of group merit selection and restricted selection among full-sib progenies of Scots pine. For. Genet. 7: 137-144. Omi, S.R. and Adams, W.T. 1985. Variation in seed set and proportions of outcrossed progeny with clones, crown position and top pruning in a Douglas-fir seed orchard. Can. J. For. Res. 16: 502-507. O'Reilly, C., Parker, W.H. and Barker, J.E. 1982. Effect of pollination period and strobili number on random mating in a clonal seed orchard of Picea mariana. Silvae Genet. 31: 90-94. Owens, J.N. 1995. Constrains to seed production: temperate and tropical trees. Tree Physiology 15: 477-484. Pakkanen, A. and Pulkkinen, P. 1991. Pollen production and background pollination levels in Scots pine seed orchards of northern Finnish origin. In: Pollen contamination in seed orchards. Ed) Dr. Lindgren, D. Proc. Mtg Nordic Group for Tree Breeding. 1991. Sweden. p. 14-21. Paule, L. 1991. Clone identity and contamination in a Scots pine seed orchard. In: Pollen contamination in seed orchards. Ed) Dr. Lindgren, D. Proc. Mtg Nordic Group for Tree Breeding. 1991. Sweden. p.6-13. 71 Paule, L. and Gömöry, D. 1992. Genetic processes in seed orchards as illustrated by European larch (Larix decidua Mill) and Scots pine (Pinus sylvestris L.). In: Proc. international conference on phytotechnique and forest management in present ecological conditions, Technical University, Zvolen. p.109-114. Paule, L., Lindgren, D. and Yazdani, R. 1993. Allozyme frequencies, outcrossing rate and pollen contamination in Picea abies seed orchards. Scan. J. For. Res. 8: 8-17. Pharis, R.P., Ross, S.D. and McMullan, E.E. 1980. Promotion of flowering in the Pinaceae by gibberellins. III. Seedling of Douglas.fir. Physiol. Plant. 50: 119-126. Philipsson, J.J. 1997. Predicting cone crop potential in conifers by assessment of developing cone buds and cones. Forestry 70: 87-96. Pollak, E. 1977. Effective population numbers and their interrelations. In: Proc. WSU conf. on Biomathematics and biostatistics, 1974. Washington State University. Portlock, F.T. 1988. Forest tree seed inspectors' manual: OECD scheme for certification of forest reproductive material moving in international trade. Information Report DPC-X-22. Forestry Canada, Ottawa, 1988. Vol. 22. 45p. Pulkkinen, P. 1994. Aerobiology of pine pollen: dispersal of pollen from non-uniform sources and impact on Scots pine seed orchards. Reports from the foundation for forest tree breeding 8. 23p. Reynolds, S. and El-Kassaby, Y.A. 1990. Parental balance in Douglas-fir seed orchards - cone crop vs. seed crop. Silvae Genet. 39: 40-42. Ritland, K. and El-Kassaby, Y.A. 1985. The nature of inbreeding in a seed orchard of Douglas fir as shown by an efficient multilocus model. Theor. Appl. Genet. 71: 375-384. Roberds, J.H., Friedman, S.T. and El-Kassaby, Y.A. 1991. Effective number of pollen parents in clonal seed orchards. Theor. Appl. Genet. 82: 313-320. Robertson, A. 1961. Inbreeding in artificial selection programmes. Genet. Res. 2: 189-194. Ross, S.D. 1978. Influences of gibberellins and cultural practices on early flowering of Douglas-fir seedling and grafts. In: Proc. 3rd World consultation on forest tress breeding, Vol. 2, Eds) Brown, A.G. & C.M. Palmberg. CSIRO. Canberra. p.9771007. Ross, S.D., Webber, J.E., Pharis, R.P. and Owens, J.N. 1985. Interaction between gibberellin A4/7 and root-pruning on the reproductive and vegetative process in Douglas-fir. I. Effects on flowering. Can. J. For. Res. 15: 341-347. Rosvall, O. 1999. Enhancing gain from long-term forest tree breeding while conserving genetic diversity. Ph.D. thesis. Swedish University of Agricultural Sciences, Umeå, Sweden. Acta Universitatis Agriculturae Sueciae, Silvestria 109. Rudin, D. and Ekberg, I. 1982. Genetic structure of open-pollinated progenies from a seed orchard of Pinus sylvestris. Silva Fenn. 16: 87-93. Rudin, D. and Lindgren, D. 1977. Isozyme studies in seed orchards. Stud. For. Suec. 139. 23pp. Rudin, D., Muona, O. and Yazdani, R. 1986. Comparison of the mating system of Pinus sylvestris in natural stands and seed orchards. Hereditas 104: 15-19. Sarvas, R. 1962. Investigations on the flowering and seed crop of Pinus sylvestris. Commun. Inst. For. Fenn. 53. 198pp. Sarvas, R. 1970. Establishment and registration of seed orchards. Folia Forestalia 89. 24p. 72 Savolainen, O. 1991. Background pollination in seed orchards. In: Pollen contamination in seed orchards. Ed) Dr. Lindgren, D. Proc. Mtg Nordic Group for Tree Breeding. 1991. Sweden. p.6-13. Savolainen, O. and Kärkkäinen, K. 1992. Effect of forest management on gene pools. New For. 6: 326-345. Savolainen, O. and Kuittinen, H. 2000. Small population processes. In: Forest conservation genetics: principles and practice. Eds) A. Young, D. Boshier and T. Boyle. CABI publishing, Unite Kingdom. p.91-100. Savolainen, O., Kärkkäinen, K., Harju, A., Nikkanen, T. and Rusanen, M. 1993. Fertility variation in Pinus sylvestris: a test of sexual allocation theory. Amer. J. Bot. 80: 1016-1020. Schmidtling, R.C. 1981. The inheritance of precocity and its relationship with growth in loblolly pine. Silvae Genet. 30: 188-192. Schmidtling, R.C. 1983. Genetic variation in fruitfulness in a loblolly pine (Pinus taeda L.). Silvae Genet. 32: 76-80. Schoen, D.J. and Stewart, S.C. 1987. Variation in male fertilities and pairwise mating probabilities in Picea glauca. Genetics 116: 141-152. Schoen, D.J., Denti, D. and Steward, S.C. 1986. Strobilus production in a clonal white spruce seed orchard: evidence for unbalanced mating. Silvae Genet. 35: 201-205. Schultz, R.P. 1971. Stimulation of flower and seed production in a young slash pine orchard. U.S. Southeastern For. Exp. Station. USDA Forest Service, SE-91, 10pp. Sedgley, M. and Griffin, A.R. 1989. Sexual reproduction of tree crops. Academic press, London. Setiawati, Y.G.B. and Sweet, G.B. 1995. Increase of seed yield and physical quality in a Pinus radiata control-pollinated meadow seed orchard. Silvae Genet. 45: 9196. Shaw, D.V. and Allard, R.W. 1982. Estimation of outcrossing rates in Douglas fir using isozyme markers. Theor. Appl. Genet. 62: 113-120. Shen, H.-H., Rudin, D. and Lindgren, D. 1981. Study of the pollination pattern in a Scots pine seed orchard by means of isozyme analysis. Silvae Genet. 30: 7-15. Silen, R.R. 1962. Pollen disposal considerations for Douglas fir. J. For. 60(11): 790795. Silen, R.R. 1963. Effect of altitude on factors of pollen contamination of Douglas-fir seed orchards. J. For. 61: 281-283. Silen, R.R and Keane, G. 1969. Cooling a Douglas-fir seed orchard to avoid pollen contamination. USDA, For. Serv., Res. Note PNW-101. 10pp. Skroppa, T. 1994. Impacts of tree improvement on genetic structure and diversity of planted forests. Silva Fenn. 28: 265-274. Skroppa, T. and Tutturen, R. 1985. Flowering in Norway spruce seed orchards. Silvae Genet. 34: 90-95. Slee, M.U. and Spidy, T. 1970. The incidence of graft incompatibility with related stock in Pinus caribaea Mor. var. hondurensis E. et G. Silvae Genet. 19: 184-187. Sluder, E.R. 1994. Gains in fusiform rust resistance and height growth in a second generation slash pine seedling orchard. Silvae Genet. 43: 41-48. Smith, D.B. and Adams, W.T. 1983. Measuring pollen contamination in clonal seed orchards with the aid of genetic markers. In: Proc. 17th SFTIC. p.69-77. 73 Sniezko, R.A. 1984. Inbreeding and outcrossing in Loblolly pine. Ph.D. thesis. Dep. of forestry, NCSU. 50pp. Sorensen, F. 1969. Embryonic genetic load in coastal Douglas-fir, Pseudotsuga menzieii var. menziesii. Am. Nat. 103: 389-398. Squillace, A.E. 1967. Effectiveness of 400-foot isolation around a slash pine seed orchard. J. For. 65: 823-824. Squillace, A.E. and Goddard, R.E. 1982. Selfing in clonal seed orchards of slash pine. For. Sci. 28: 71-78. Squillace, A.E. and Kraus, J.F. 1963. The degree of natural selfing in Slash pine as estimated from albino frequencies. Silvae Genet. 12: 46-50. Squillace, A.E. and Long, E.M. 1981. Proportion of pollen from nonorchard clones. In: Pollen managmenet handbook. Ed) E.C. Franklin. US. Dep. Agric. Agric. Handb. No. 586. p.15-19. Stewart, S.C. 1994. Simultaneous estimation of pollen contamination and pollen fertilities of individual trees in conifer seed orchards using multilocus genetic data. Theor. Appl. Genet. 88: 593-596. Stoehr, M.U. and El-Kassaby, Y.A. 1997. Levels of genetic diversity at different stages of the domestication cycle of interior spruce in British Columbia. Theor. Appl. Genet. 94: 83-90. Stoehr, M.U., Orvar, B.L., Vo, T.M., Gawley, J.R., Webber, J.E. and Newton, C.H. 1998. Application of a chloroplast DNA marker in seed orchard management evaluations of Douglas-fir. Can. J. For. Res. 28: 187-195. Stoehr, M.U., Webber, J.E. and Painter, R.A. 1994. Pollen contamination effects on progeny from an off-site Douglas-fir seed orchard. Can. J. For. Res. 24: 21132117. Sweet, G.B. 1995. Seed orchards in development. Tree Physiology 15: 527-530. Toda, R. 1964. A brief review and conclusion of the discussion on seed orchards. Silvae Genet. 13: 1-4. van Buijtenen, J.P. 1971. Seed orchard design, theory and practice. Proc. 11th SFTIC, Atlanta, Ga. 1971. p.197-206. Varghese, M., Nicodemus, A., Nagarajan, B., Siddappa, K.R.S., Bennet, S.S.R. and Subramanian, K. 2000. Seedling seed orchards for breeding tropical trees. Scroll press, Coimbatore, India. 126pp. ISBN 81-900346-1. Varnell, R.J., Squillace, A.E. and Bengston, G.W. 1967. Variation and heritability of fruitfulness in slash pine. Silvae Genet. 16: 125-128. Verrier, E., Colleau, J.J. and Foulley, J.L. 1991. Methods for predicting response to selection in small populations under additive genetic models: a review. Livest. Prod. Sci. 29: 93-114. Wang, T., Hagqvist, R. and Tigerstedt, P.M.A. 1999. Inbreeding depression in three generations of selfed families of silver birch (Betula pendula). Can. J. For. Res. 29: 662-668. Wang, X.R., Lindgren, D., Szmidt, A.E. and Yazdani, R. 1991. Pollen migration into a seed orchard of Pinus sylvestris L. and the methods of its estimation using allozyme markers. Scand. J. For. Res. 6: 379-385. Wei, R.-P. 1995. Predicting genetic diversity and optimising selection. Ph.D. thesis. Swedish University of Agricultural Sciences, Umeå, Sweden. 74 Wheeler, N. and Jech, K. 1986. Pollen contamination in a mature Douglas-fir seed orchard. In: Proc. IUFRO conf. on Breeding theory, progeny testing and seed orchards. Williamsburg, Oct 13-17, Virginia. p.160-171. Wheeler, N.C. and Jech, K.S. 1992. The use of electrophoretic markers in seed orchard research. New For. 6: 311-328. Wheeler, N.C., Masters, C.J., Cade, S.C., Ross, S.D., Keeley, J.W. and Hsin, L.Y. 1985. Girdling: an effective and practical treatment foe enhancing seed yield in Douglas-fir seed orchards. Can. J. For. Res. 15: 505-510. Williams, C.G. and Savolainen, O. 1996. Inbreeding depression in conifers: implication for breeding strategy. For. Sci. 42: 102-117. Williams, C.G., Hamrick, J.L. and Lewis, P.O. 1995. Multiple-population versus hierarchical conifer breeding programs: a comparison of genetic diversity levels. Theor. Appl. Genet. 90: 584-594. Wright, S. 1931. Evolution in Mendelian populations. In: Genetics, Brooklyn Botanic Garden, Brooklyn, New York. p.97-158. Wright, J.W. 1953. Pollen-dispersion studies: some practical application. J. For. 51: 114-118. Xie, C.Y. and Knowles, P. 1992. Male fertility variation in an open-pollinated plantation of Norway spruce (Picea abies). Can. J. For. Res. 22: 1463-1468. Xie, C.Y. and Knowles, P. 1994. Mating system and effective pollen immigration in a Norway spruce (Picea abies (L.) Karst) plantation. Silvae Genet. 43: 48-52. Xie, C.Y., Yeh, F.C., Dancik, B.P. and Strobeck, C. 1991. Joint estimation of immigration and mating system parameters in gymnosperms using the EM algorithm. Theor. Appl. Genet. 83: 137-140. Yazdani, R. and Lindgren, D. 1991. Variation of pollen contamination in a Scots pine seed orchard. Silvae Genet. 40: 243-246. Yazdani, R., Hadders, G. and Szmidt, A.E. 1986. Supplemental mass pollination in a seed orchard of Pinus sylvestris L. investigated by isozyme analysis. Scand. J. For. Res. 1: 309-315. Yazdani, R., Lindgren, D., Seyedyazdani, F., Pascual, L. and Eriksson, U. 1995. Flowering, phenology, empty seeds and pollen contamination in a clonal seed orchard of Pinus sylvestris in northern Sweden. In: Population genetics and genetic conservation of forest trees. Ed) Baradat,, W.T. Adams & G. MullerStarck. SPB Academic Publishing, Amsterdam, The Netherlands. p.309-319. Yeh, F.C. 2000. Population genetics. In: Forest conservation genetics - principles and practice. Eds) A. Young, D. Boshier and T. Boyle. CABI publishing. London. p.21-37. Zobel, B. and Talbert, J. 1984. Applied forest tree improvement. John Wiley & Sons, New York, USA. 505pp. Zobel, B.J., Barber, J., Brown, C.L. and Perry, T.O. 1958. Seed orchards; their concept and management. J. For. 56: 815-825. 75 Acknowledgements I would greatly appreciate my supervisor, Prof. Dag Lindgren for accepting and teaching me as a Ph.D. student at the Department of Forest Genetics and Plant Physiology in the Swedish University of Agricultural Sciences (SLU) and also for supervising my doctoral thesis. He has given me excellent guidance through four years in my study in Sweden. My thanks have to go to Drs. Adolfo Bila and Anni Harju for their collaboration and valuable discussion. Also, many thanks to my colleagues, Dr. Derius Danusevicius, Dr. Seog-Gu Son, Mrs. Thuy Olsson, Mr. Torgny Persson, Mr. Daoqun Zang, and ex-colleagues, Dr. Erik Andersson, Dr. Huen-Lin Li, Dr. Leopoldo S. Rodriguez, Dr. Yong-Qi Zheng and Mr. Seppo Ruotsalneinen. It has been a pleasure working with such nice colleagues. I wish to deeply thank Dr. Tim J. Mullin at NCSU in the USA, Dr. Erik D. Kjær in Denmark, Profs. Jung Oh Hyun and Min Sup Chung in Korea, and Dr. Yill Sung Park in Canada for their cooperation, comments and reviewing at the stage of manuscripts. I also thank Dr. Teijo Nikkanen from the Finnish Forest Research Institute and Mr. Curt Almqvist from the Swedish Forest Research Institute (SkogForsk) for their co-operation in paper VII and fruitful discussion. Also, many thanks to people from SkogForsk at Sävar. It was a particular pleasure for me to have encouragement and congratulations from Dr. Gene Namkoong on completing the piece of my work. I would like to sincerely acknowledge all colleagues (especially Drs. Alfred E. Szmidt, Anders Fries, Dag Rudin, Jan-Erik Nilsson and Katarina Lindgren) at this department for providing me warm friendship and for sharing seminars and discussion during my time in Umeå. I will keep in mind your cooperation, Estelle, Laura, Maria, Wan, Xiao-Ru, Tongming and Vikram. Gertrud Lestander, Maria Lindgren and Stefan Löfmark have helped and assisted me like a mother, a sister and a brother, whenever and whatever I needed. My employer, the Korea Forest Research Institute, the Swedish Kempe Foundation and Föreningen Skogsträdsförädling have supported my study grants. I thank my boss, Dr. Wan-Yong Choi, for encouraging me to be a Ph.D. student in the field of seed orchard research. I am especially grateful to my parents, brother and sisters for their unconditional support and understanding. Finally, my infinite thank goes to my wife, Eun-Joo Yang and to my son, Young-Jong (Swedish name, “Sam”) Kang for their patience and tolerance of the long, dark, cold Swedish winter. How could I live without you! Many kisses and hugs to both of you! I want to dedicate this doctoral thesis to my grandmother and father-in-law who passed away before this thesis is finished. 76