Widths of Spectral Lines (continued)
advertisement

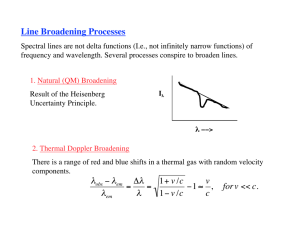
Widths of Spectral Lines (continued) Pressure Broadening This cause of line broadening may be regarded as being due to random collisions between atoms, which disturb internal interactions and shorten the lifetime. e.g., Lorentz model (see Corney, p.241) This model assumes that an atom excited at time t = 0 radiates classically until a collision occurs. The decaying wavetrain is then terminated – i.e., the radiation is cut off early, hence the lifetime is apparently shortened. As with the natural linewidth, the lineshape is a Lorentzian. L( ) L 2 1 ( 0 ) L 2 2 2 with FWHM L 1 and c mean time between collisions. c For example, consider a mercury lamp running at 150C – this gives a vapour pressure. p, of 2.8 mmHg. For the collisions, a hard sphere approximation and atoms of diameter d can be assumed. Atoms collide only if the impact parameter d To obtain the mean time between collisions, the number of collisions per second, c is required. Consider an atom travelling with average speed vrms. Then, the “volume”, V, swept in 1 second by the atom, within which a collision can occur is: V d 2v rms Atom “sweeping” a cylinder of diameter d. Atom undergoing collision Atom within collision region Atom outside collision region. If there are n atoms per unit volume, the collision frequency is simply this density multiplied by the volume swept by an atom per second: c nd 2v rms Hence, the mean time between collisions is: (substituting for vrms from Maxwell-Boltzmann statistics) 1 m c c nd 2 3kT 1 1 2 For mercury, m = 200 a.u. p In the example of the mercury lamp, n kT 6.31 10 22 m3 and v rms 229 ms1 If d 4.5 Å, then c 109 ns or, l 2.93 MHz. This is about 25% of the natural linewidth, but for higher pressures this type of broadening is usually larger. Sometimes, pressure broadening is referred to as homogeneous broadening, since if n is reduced by removing a selection of atoms, the whole line narrows. Also, l is independent of the frequency of the spectral line. Doppler Broadening Unlike the other forms of broadening described above, Doppler broadening does not involve intra- or inter-atomic interactions. Rather, it is due to the motions of the atoms with respect to an observer. Atom moving towards observer – light is blue shifted. Atom moving away from observer – light is red shifted. To find the form of the distribution, it is first necessary to consider the velocity distribution along the line of sight of the observer, vx. What is the probability, P(vx) of the velocity being in the range vxvx + dvx? From Maxwell-Boltzmann statistics, P(v x )dv x e mv x2 2 kT dv x The Doppler shift is given by 0 Doppler broadened line profile is: vx where 0 is the line centre frequency. Hence, the c m c 2 ( 0 ) 2 D( )d D 0 exp d where 02 2kT c m D0 0 2kt 1 2 , and the FWHM, D 2 D0 is a 0 2kT ln(2) c m normalising constant, 1 2 . For a gas of atomic/molecular mass M in a.u. at a temperature T K, the above reduces to: D 0 7.16 10 7 T M The form of this profile is Gaussian – somewhat different from the Lorentzian profiles of the previous cases. This reflects its different origin – an effect related to the motions of atoms rather than decays of radiation. As an example, in the mercury lamp discussed earlier; M = 200 a.u., T = 150ºC and 0 = 546 nm. Then, D = 570 MHz – ca. 50 the natural line width. This is usually the dominant source of broadening in gas and vapour sources. Notice also that the amount of broadening depends on the centre frequency of the spectral line. Doppler broadening is sometimes called inhomogeneous broadening since each atom contributed its own frequency to the profile. Hence, removal of a (non-random) sample of atoms (e.g., by staturating a transition at a particular frquency) will distort the line shape. A consequence is Doppler-free, or Saturated absorption spectroscopy. Absorption of Radiation and Absorption Coefficients On passing through a medium, radiation may be lost via a number of mechanisms. For example: Scattering Absorption x Absorption The final mechanism leads to an increase in the internal energy of the medium, through, for example, electronic excitation of constituent atoms. I0 Scattering I To quantify these losses, consider a monochromatic, collimated beam incident on small segment of the medium of thickness x, over which the intensity changes by an amount, I = I(x+x) - I(x) . Then, if the medium is homogeneous, we can write: I( x x ) I( x ) I( x )x (i.e., the change in intensity is proportional both to the incident intensity and the amount of absorber) I(x) Hence, i I( x )x Writing as a differential equation, dI(x ) I(x ) dx I(x+x) x This is clearly the form of an exponential, hence I( x ) I 0 e x where is the absorption coefficient. In the context of a laser, we can ask: How is the absorption coefficient related to the Einstein coefficients? What is it's wavelength dependence? How is it affected by line shapes? Can a situation be arrived at where the incident radiation gains rather than loses intensity - i.e., amplification?