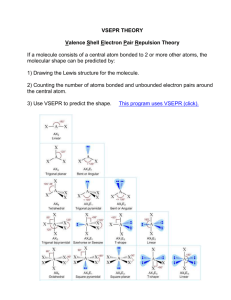
Drawing Lewis Structures
advertisement

Drawing Lewis Structures The following step-by step instructions are designed to produce a correct Lewis Structure for any molecular assembly (neutral molecule or polyatomic ion) which contains only Main Group elements joined by two-atom shared-pair covalent bonds. 1. 2. 3. 4. 5. 6. 7. Connectivity - arrange bonded atoms VSE - count valence shell electrons BP - assign bond pairs PLP - assign lone pairs to peripheral atoms CLP - assign lone pairs to central atoms Rearrange - find best Lewis Structure(s) [formal charge] Extra - assigning oxidation numbers These rules are not appropriate for free radicals or molecular assemblies which contain multicenter bonds or transition metals. Step 1: Connectivity - arrange the atomic symbols so that covalently bonded atoms are contiguous. Commentary Some general rules and definitions: 1. the number of covalent bonds an atom forms is called its valence. 2. Some atoms have fixed valence. For example: H = 1, C = 4, F = 1. 3. Some atoms have variable valence. For example: O = 2 (sometimes 3), B, N = 3 (sometimes 4). 4. an atom bonded to only one other atom is peripheral (monovalent atoms such as H and F are always peripheral). 5. an atom bonded to two or more other atoms is central. Often, the formula is written to indicate connectivity. For example: HCN = H bonded to C, C bonded to N, H and N are not bonded. CH3OCH3 = three H bonded to C1, C1 bonded to O, O bonded to C2, C2 bonded to three H. CH3CH2OH = three H bonded to C1, C1 bonded to C2, C2 bonded to two H and O, O bonded to H. Otherwise, as a general rule, the least electronegative elements (if not monovalent) are central, the most electronegative elements are peripheral. For example: CO32- = 3 peripheral O bonded to central C PO43- = 4 peripheral O bonded to central P H3O+ = 3 monovalent (therefore peripheral) H bonded to central O Note that the order or geometric arrangement of symbols written on the page is irrelevant as long as bonded atom pairs are contiguous. The final Lewis Structure represents onlythe approximate electronic disribution,not the actual 3-dimensional arrangement of the atoms. back to top Step 2: VSE - Count the total number of Valence Shell Electrons; divide these VSE into pairs. Commentary 1. Sum the number of valence shell electrons of each atom; the number of valence shell electrons for an atom is equal to its group number 2. Subtract the charge on the assembly 3. The total should be even (these rules do no apply to assemblies with an odd number of electrons) Examples: HCN = 1+4+5-0 = 10 VSE = 5 VSE pairs C2H6O = 2(4)+6(1)+6-0 = 20 VSE = 10 VSE pairs CO32- = 4+3(6)-(-2) = 24 VSE = 12 VSE pairs PO43- = 5+4(6)-(-3) = 32 VSE = 16 VSE pairs H3O+ = 3(1)+6-(+1) = 8 VSE = 4 VSE pairs back to top Step 3: Assign BP - Place one VSE pair of electrons between each bonded pair of atoms. Commentary Connect each contiguous pair of atoms with one of the VSE pairs; each Bond Pair is shown as a line. Examples: HCN = 5 VSE pairs - 2 BP = 3 pairs remaining C2H6O = 10 VSE pairs - 8 BP = 2 pairs remaining CO32- = 12 VSE pairs - 3 BP = 9 pairs remaining PO43- = 16 VSE pairs - 4 BP = 12 pairs remaining H3O+ = 4 VSE pairs - 3 BP = 1 pair remaining back to top Step 4: Assign Peripheral LP - Place up to three VSE pairs on each peripheral atom. Commentary Distribute the VSE pairs remaining after Step 3 among the peripheral atoms as Lone Pairs (H cannot accept lone pairs; see the Rule of Orbitals below). At this stage, no peripheral atom may have more than 4 VSE pairs (1 BP + 3 LP). Examples HCN : of 3 VSE pairs, all are assigned to N; no VSE pairs remain. C2H6O : of 2 VSE pairs, none are assigned (since all peripheral atoms are H); 2 VSE pairs remain. CO32- : of 9 VSE pairs, 3 are assigned to each O; no VSE pairs remain. PO43- : of 12 VSE pairs, 3 are assigned to each O; no VSE pairs remain. H3O+ : 1 pair, not assigned (since all peripheral atoms are H); 1 VSE pair remains. back to top Step 5: Assign Central LP - Place any remaining VSE pairs as Lone Pairs on central atom(s) according to the Rule of Orbitals. Commentary The Rule of Orbitals: the total number of lone pairs and bond pairs (LP+BP) associated with an atom cannot exceed the number of Valence Shell Orbitals (VSO = n2, where n is the row of the Periodic Table in which that atom resides). n = 1 (H): maximum VSE pairs (LP+BP) = VSO = 1; n = 2 (B, C, N, O, F): maximum VSE pairs (LP+BP) = VSO = 4 ("octet rule") n = 3 ((Al, Si, P, S, Cl): maximum VSE pairs (LP+BP) = VSO = 9; etc. Examples C2H6O = 2 pairs, both assigned to O since each C already has 4 BP. H3O+ = 1 pair, assigned to O. While C,N,O and F always fill their four valence shell orbitals with Lone Pairs and/or Bond Pairs, B often does not. Thus, these four atoms always obey the "octet rule" (the only atoms on the periodic chart which always do!), but some compounds with B have an empty valence shell orbital and are called "electron deficient". Furthermore, third row elements (e.g., Al, Si, P, S, Cl) often have more than four valence shell orbitals filled with Lone Pairs and/or Bond Pairs; this is called (illogically) "expanded valence". Obviously, elements from the fourth and higher rows can also exhibit "expanded valence". The tendency of most main group elements (except H) is to form molecular assemblies which fill at least the first four orbitals (s and p subshells); this tendency, plus the ubiquity of assemblies which contain C, N and O, has led to the (over) emphasis on the "octet rule". back to top Step 6: Rearrange VSE Pairs - If necessary, push electron pairs according to the Rule of Orbitals and the Principle of Electroneutrality. Commentary Principle of Electroneutrality: each atom in a covalent molecular assembly has a formal charge close to zero. Formal Charge: FC = (Group Number) - (Bond Pairs) - 2(Lone Pairs) Electron Pushing: formally changing a lone pair into a bond pair, or vice versa, while retaining association with the atom. Examples HCN Original Lewis Structure H: FC = 1-1-2(0) = 0; H: rule of orbitals satisfied (1 orbital, 1 VSE pair); C: FC = 4-2-2(0) = +2; C: rule of orbitals not satisfied (4 orbitals, only 2 VSE pairs; it must be associated with 4 VSE pairs); N: FC = 5-1-2(3) = -2; N: rule of orbitals satisfied (4 orbitals, 4 VSE pairs) Rearranged Lewis Structure Push two lone pairs on N into C-N bonding position, creating a C-N triple covalent bond. H: FC = 0; C: FC = 4-4-2(0) = 0; N: FC = 5-3-2(1) = 0; All atoms: rule of orbitals satisfied. C2H6O (both isomers) Original Lewis Structures H (all): FC = 1-1-2(0) = 0; H (all): rule of orbitals satisfied; C (all): FC = 4-4-2(0) = 0; C (all): rule of orbitals satisfied; O: FC = 6-2-2(2) = 0; O: rule of orbitals satisfied; the original Lewis Diagrams (produced with rules 1-5) are correct, no rearrangement necessary. CO32Original Lewis Structure O (all): FC = 6-1-2(3) = -1; O (all): rule of orbitals satisfied; C: FC = 4-3-2(0) = +1; C: rule of orbitals not satisfied; it must be associated with 4 VSE pairs. Rearranged Lewis Structure Push a lone pair on any of the three O into bonding position, creating one CO double bond. For each of the three Lewis Structures thus produced: O (single bond): FC = -1; O (double bond): FC = 6-2-2(2) = 0 (average formal charge on each oxygen = -2/3); C: FC = 4-4-2(0) = 0; all atoms: rule of orbitals satisfied; The set of three rearranged Lewis Structures correctly depicts the resonance in this ion. PO43O (all): FC = 6-1-2(3) = -1; O (all): rule of orbitals satisfied; P: FC = 5-4-2(0) = +1; P: rule of orbitals satisfied (P can accomodate up to 9 VSE pairs); Push a lone pair on any of the four O into bonding position, creating one PO double bond. For each of the four Lewis Diagrams thus produced: O (single bond): FC = -1; O (double bond): FC = 0 (average formal charge on each oxygen = -3/4); P: FC = 5-5-2(0) = 0; all atoms: rule of orbitals satisfied; The set of four rearranged Lewis Diagrams correctly depicts the resonance structures of this ion. H3 O+ H (all): FC = 0; H (all) rule of orbitals satisfied; O: FC = 6-3-2(1) = +1; O: rule of orbitals satisfied. The original Lewis Structure is correct. The formal charge of +1 on oxygen is reduced by electronegative induction, so each H atom attains a slight positive charge while the charge on the O atom approaches 0. back to top Extra: Oxidation Numbers General Rules 1. Each atom in an element has ON = 0 Examples: He(g), H2(g), Br2(l), P4(g), S8(s), Fe(s) 2. In ionic compounds, molecules and polyatomic ions a. Oxygen: ON(O) = -2 usually Examples: Na2SO3, SO3, SO42-; but in Na2O2 and H2O2, ON(O) = -1 b. Hydrogen with a metal: ON(H) = -1 Examples: NaH, CaH2 c. Hydrogen with a non-metal: ON(H) = +1 Examples: CH4, H2O, HPO42d. Fluorine: ON(F) = -1 always 3. The sum of all oxidation numbers is equal to the charge on the compound, molecule or ion 4. For any Main Group atom, maximum ON = Group Number Examples: C (+4), P (+5), Se (+6), Br (+7), Xe (+8) 5. For any Main Group atom, minimum ON = Group Number - 8 Examples: C (-4), P (-3), Se (-2), Br (-1), Xe (0) Lewis Diagrams The oxidation number for an individual atom in a Lewis Diagram is calculated as follows: Assign both electrons of a lone pair to the valence shell of the atom. Assign both electrons of a bond pair to the valence shell of the most electronegative atom. Examples o H-F => H :F o C-O => C :O o C-H => C: H If both bonded atoms are identical (and therefore have the same electronegativity), assign one bonding electron to the valence shell of each atom. Examples o C-C => C. .C o C=C => C: :C VSE = the total number of electrons (lone pairs, bond pairs, and single electrons) thus assigned to the valence shell of the atom. ON = Group Number - VSE Check: the sum of the oxidation numbers of all atoms in the Lewis Structure = the total charge on the Lewis Structure. Examples: HCN: C is more electronegative than H, so it is assigned the H-C bond pair; N is more electronegative than C, so it is assigned all three C-N bond pairs. Then ON(H) = 1-0 = +1 ON(C) = 4-2 = +2 ON(N) = 5-8 = -3 (+1) + (+2) + (-3) = 0 CH3OCH3: O is more electronegative than C, so it is assigned both C-O bond pairs; C is more electronegative than H, so both C(1) (the left-most C atom) and C(2) (the right-most C atom) are assigned every C-H bond pair (both C atoms are identical). Then ON(H) = 1-0 = +1 ON(C(1)) = ON(C(2)) = 4-6 = -2 ON(O) = 6-8 = -2 6(+1) + 2(-2) + (-2) = 0 Note that the oxidation number of both C atoms is equal to the value obtained by applying the General Rules. CH3CH2OH: O is more electronegative than either C or H, so O is assigned both the C-O and O-H bond pairs; C is more electronegative than H, so both C(1) (the left-most C atom) and C(2) are assigned every C-H bond pair. The C-C bond pair is split between the two C atoms. Then ON(H) = 1-0 = +1 ON(C(1)) = 4-7 = -3 ON(C(2)) = 4-5 = -1 ON(O) = 6-8 = -2 6(+1) + (-3) + (-1) + (-2) =0 Note that the average oxidation number of the two C atoms is equal to the value obtained by applying the General Rules. CO32-: O is more electronegative than C, so each O is assigned the C-O bond pair. Then ON(O) = 6-8 = -2 ON(C) = 4-0 = +4 3(-2) + (+4) = -2 It is left as an exercise for the reader to perform these calculations on the resonance structures. PO43-: O is more electronegative than P, so each O is assigned the P-O bond pair. Then ON(O) = 6-8 = -2 ON(P) = 5-0 = +5 4(-2) + (+5) = -3 It is left as an exercise for the reader to perform these calculations on the resonance structures. H3O+: O is more electronegative than H, so O is assigned all of the H-O bond pairs. Then ON(H) = 1-0 = +1 OH(O) = 6-8 = -2 (+1)+(+1)+(+1)+(-2) = +1