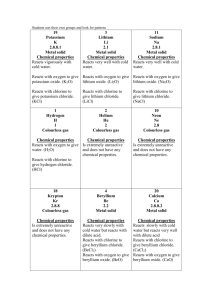
Chemistry
advertisement
Cardiff International School Dhaka (CISD) Lost Class Make Up Assignment Class: 8A Subject: Chemistry th Date: 8 (Sunday)-14th (Saturday) March 2015 Total Mark- 20 Instructions: All of your assignment must be done in A4 size paper. Mention your Name, Class, Roll and Section clearly on the top sheet of your assignment. Submission Deadline: Saturday 14th February 10.00 AM to the respective subject teacher. The deadline is strict. Name:......................................................................................................................................................... Class: ......................... Roll: ........................ Sec: .................. Teacher: ............................................. Sunday-Saturday 8-14 March 2015 Lesson: Chapter-16: The periodic table Chapter-17: Energy from chemicals Task: Revise the chapters carefully mentioned above and answer the following questions. Worksheet/Exercise: Q1. Photosynthesis is an endothermic reaction. (a) Explain, in terms of the energy changes that occur during bond breaking and bond making, why photosynthesis is an endothermic reaction. (b) Complete the energy profile diagram for photosynthesis. [2] On your diagram label the • products, • enthalpy change for the reaction, ΔH, • activation energy, Ea. [3] (c) What are the criteria of a good fuel? [3] Q2. a) State 2 properties that are used to arrange elements in the periodic table. [2] b) Use three elements from Group II of the periodic table to illustrate the trend in i) physical property ii) chemical property [4] Q3. Group-I Metals is highly reactive metal and form variety of compounds which are sparingly soluble in water. Describe the reactivity of potassium metal with water. Give necessary reaction to support your answer. [3] Q4. Hydrogen reacts with chlorine as shown by the equation below. H2 (g) + Cl2 (g) ------------------> 2HCl (g) ∆H = -184 KJ Calculate the energy change when 14.2 g of chlorine reacts completely with hydrogen. [3] Text Book/Reference Book: Chemistry Matters, Page No. 284-297, 302-313. Chapter – 16, 17 Answer keys: Please do not see the answer keys before you do the work by yourself. Once you have done all the work by yourself- you can match your answers with the answer options given below. In some cases you answer might be different from what is given below but that might be correct as well. The answer options given below for your understand only. These answer keys are the guidelines only, you may come out with different set of right answers in line with these options. Q1. The following reaction occurs in photosynthesis. 6CO2 + 6 H2O -----------------------------> C6H12O6 + 6O2 ∆H= +2802.5 kJ Q2. Group II elements are Be, Mg, Ca, Sr, Ba. Q3. Potassium metal reacts very rapidly with water to form a colourless solution of potassium hydroxide (KOH) and hydrogen gas (H2). Q4. 1 mole of chlorine (70.9 g) reacts in this reaction. Help Lines: For any assistance, please contact 1. Coordinator: 2. Chemistry Teacher: Naznin Nahar Nishi, 8801716310008,naznin_buet@yahoo.com Jobaida Akther, juibmbdu@yahoo.com 3. Principal Head of School: G.M.Nizam Uddin, +88-01622181818, gmnu302@yahoo.com