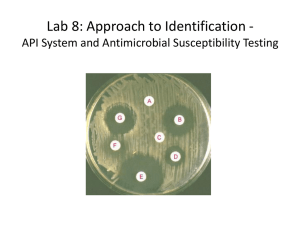
Sensitivity testin
advertisement

LABORATORY #12 Antibiotic Susceptibility Testing Laboratory #12 Antibiotic Susceptibility Testing Skills= 15 Points Objective: At the completion of this laboratory, the student will be able to: 1. Correctly set up and read Kirby-Bauer antibiotic sensitivity tests on Staphylococcus aureus and E. coli. 2. Correctly set up and interpret an Etest. 3. Discuss the principle behind the Etest. 4. Discuss the principle behind the MIC. Materials: Two 100 mm Mueller-Hinton plates Plate cultures of Staphylococcus aureus and E.coli Sterile 0.85% saline Sterile Swabs McFarland 0.5 turbidity standard Antibiotic sensitivity disks Antibiotic disk dispenser Incubator Forceps References: 1. Mahon and Manuselis, Textbook of Diagnostic Microbiology, Third Edition, Chapter 13 2. Clinical and Laboratory Standards Institutes (formerly NCCLS) Standards for Antimicrobial Susceptibility (http://www.clsi.org) Discussion: The traditional laboratory test for in vitro antimicrobial susceptibility has been the antimicrobial disk-agar diffusion procedure, the so-called disk method. Its simplicity, speed of performance, economy, and reproducibility, (under standardized conditions) have made it useful. Filter paper disks are impregnated with various antimicrobial agents of specific concentrations and are carefully placed on an agar culture plate that has been inoculated with a standardized culture of the bacterium to be tested. The broth culture is standardized by comparing the turbidity of the culture to the turbidity of a 0.5 McFarland standard, a turbid solution made by mixing barium chloride (BaCl2) and sulfuric acid (H2SO4). The antibiotic disks are usually kept refrigerated to maintain viability. It is necessary to allow the disks to come to room temperature before placing them on the inoculated agar to avoid cold shocking the organism. The disks should be placed on the inoculated agar within 15 minutes so that the organism has not MLAB 2434 – Laboratory 12 – Page 1 LABORATORY #12 Antibiotic Susceptibility Testing had a chance to begin growing and to avoid a false negative reaction. The plate is incubated overnight and observed the following day for a zone of growth inhibition around the disk containing the agent to which the organism is susceptible. Antibiotics causing zones of inhibition require that the zone size be measured and interpreted in order to determine the relative susceptibility or resistance of an organism. The size of the zone of inhibition denotes the concentration point at which the antibiotic concentration fails to inhibit the growth of the bacterium. The diameter of the zone of inhibition directly relates to the MIC. The larger the diameter of the zone of inhibition for a given antibiotic and organism, the more susceptible the organism. The resistant organism will have a smaller zone size. Antibiotic disks with no zone of inhibition indicate that the antibiotic did not inhibit the growth of the organism even at the highest concentration and the organism is resistant to the antibiotic. The disk diffusion method has been standardized through the work of Kirby and Bauer. Mueller-Hinton agar is chosen for routine susceptibility testing since it shows good batch-tobatch uniformity and is low in tetracycline and sulfonamide inhibitors. With the addition of animal blood, it will support the growth of most fastidious pathogens. Kirby-Bauer Method Procedure: 1. Working in pairs, each person will perform one Kirby-Bauer set-up. 2. Using a sterile swab, one person will select a well-isolated morphologically identical colonies of S. aureus from a plate of 18-24 hour growth on non-selected agar. The other partner will select a well-isolated morphologically identical colonies of E. coli from a plate of 18-24 hour growth on non-selected agar. 3. Transfer growth to a tube of sterile 0.85% saline. 4. Emulsify and mix well. 5. Adjust turbidity using sterile saline to equal a 0.5 McFarland standard. Hold the two tubes side by side up to a light source to compare turbidity. 6. Within 15 minutes of inoculum preparation, dip a sterile cotton-tipped swab into the inoculums suspension and swirl to saturate the swab. 7. Press and rotate the swab several times against the side of the tube to remove excess liquid. 8. Inoculate the Mueller-Hinton plate by spreading the organism suspension over the entire surface with the swab. Rotate the plate approximately 90 degrees, and swab the entire surface with the same swab. Rotate the plate another 90 degrees and swab the surface a final time to ensure an even distribution of inoculum. 9. Allow the plate to dry at least 1 minute with the lid in place. 10. Open the lid, then apply the antibiotic sensitivity discs with the dispenser. The instructor will demonstrate this technique. 11. Close the plate and tap the bottom of the plate lightly against the edge of a tabletop to ensure contact between the agar and the disks. 12. Place the plate in the 35°-37°C, non- CO2 incubator overnight. MLAB 2434 – Laboratory 12 – Page 2 LABORATORY #12 Antibiotic Susceptibility Testing 13. Using a metric ruler, measure the zones of inhibition (no growth zones) around each antibiotic sensitivity disk from edge of growth through the disk to the opposite edge of growth. 14. Record the zone sizes in millimeters (mm) on the Kirby-Bauer report form. 15. Then, indicate on the report form if the zone indicates the organism is “sensitive”, “intermediate”, or “resistant” to each antibiotic, based on the Kirby-Bauer Standard Antibiotic Sensitivity Zone chart at the end of this laboratory exercise. E Test System Principle: The E-test is a quantitative MIC method for testing the antimicrobial susceptibility of fastidious and non-fastidious organisms. Etest is used routinely for testing the MIC of Streptococcus pneumonia and strep viridians group isolates recovered from blood cultures of endocarditis patients and other streps as requested. One benefit of the Etest system is that the antimicrobial menu is easily customized, however, it is expensive so it may not be extensively used. The E-test strip is an inert plastic strip which has a numerical MIC scale printed on one side and an antibiotic concentration gradient dried on the other side. The strips are applied to a standardized inoculum of an organism swabbed onto the surface of an agar plate. The antimicrobial agent diffuses into the agar, and a stable continuous antibiotic concentration gradient is established along the sides of the strip. After incubation, an elliptical zone of inhibition forms. The MIC is read where organism’s growth intersects the E-strip scale. Materials: Etest strips of designated test antimicrobials Plate culture of S. pneumoniae Sterile cotton-tipped swabs McFarland 0.5 standard Sterile pipettes One 150 mm Mueller Hinton sheep blood agar (MHB plates) 0.85% saline Forceps Incubator, 35oC, CO2 Procedure: 1. Allow Etest strips to equilibrate to room temperature for 30 minutes prior to use. 2. Allow Mueller Hinton sheep blood agar plates to equilibrate to room temperature. 3. Using a sterile swab, select well-isolated morphologically identical colonies from a plate of 18-24 hour growth on non-selected agar. 4. Transfer growth to a tube of sterile 0.85% saline. 5. Emulsify and mix well. 6. Adjust turbidity using sterile saline to equal a 0.5 McFarland standard. Hold the two tubes side by side up to a light source to compare turbidity. a. NOTE: When testing mucoid Strep pneumonia or other mucoid or fastidious organisms, the inoculum should be equivalent to McFarland #1 standard. MLAB 2434 – Laboratory 12 – Page 3 LABORATORY #12 Antibiotic Susceptibility Testing 7. Within 15 minutes of inoculum preparation, dip a sterile cotton-tipped swab into the inoculum suspension and swirl to saturate the swab. 8. Press and rotate the swab several times against the side of the tube to remove excess liquid. 9. Inoculate the plate by spreading the organism suspension over the entire surface with the swab. Rotate the plate approximately 90 degrees, and swab the entire surface with the same swab. Rotate the plate another 90 degrees and swab the surface a final time to ensure an even distribution of inoculum. 10. Allow the inoculum to dry on the plates for 10-15 minutes before proceeding. This step is critical to proper performance. 11. Using forceps or fingers, grip the handle of the strip (E label end) and remove from the package. DO NOT touch the antibiotic gradient. 12. Holding the strip at an angle to the plate, place the bottom edge of the strip against the agar surface with the MIC scale facing upwards and the “E” label end pointing to the outer edge of the plate. 13. Gradually allow the strip to come in contact with the agar, by anchoring the end with a swab and slowly lowering the strip. 14. Make sure the strip is in complete contact with the agar surface. Remove air pockets underneath by gently pressing with a swab, moving from the minimum concentration upwards. Small bubbles under the strip will not affect the results. Once applied, the position of the strip cannot be changed due to the immediate release of the antibiotic from the underside of the strip on the agar. 15. When using a 150-mm plate, place up to six Etest strips on the plate. Place the strips an equal distance apart, radiating from the center of the plate like spokes of a wheel with the “E” end of the strip pointing outwards. 16. If the strip is accidentally placed upside down, carefully pick it up by the “E” end, turn it over, and position it properly on the agar surface. 17. If the strip touches the surface of the bench or another object, it can still be used as long as it does not come in contact with moisture. 18. Invert the plates and incubate for 18-24 hours in a 35OC CO2 incubator within 1 hour of strip application. Interpretation/Reading plates: 1. Read plates only if the lawn of growth is confluent or nearly confluent. 2. Remove the lid and use reflected light to read the MIC at the point where the growth intersects the strip. 3. If the growth does not intersect the strip, record the MIC as less than the lowest concentration of antimicrobial agent on the Etest strip. 4. If growth occurs along the entire strip (no ellipse seen), record the MIC as greater than the highest value on the scale. 5. If growth intersects the strip, record the MIC as the numerical value at the point of intersection. For MICs that fall between markings, record the result as the higher value. 6. Enter the numerical MIC value in the report form for the designated Etest strip. 7. Using the MIC value, and the chart below, interpret as “S, I, or R” based on organism specific database definitions. MLAB 2434 – Laboratory 12 – Page 4 LABORATORY #12 Antibiotic Susceptibility Testing Interpretation for Streptococcus Pneumoniae *NCCLS interpretative standards for MICs(ug/mL) S Levofloxicin Erythromycin Vancomycin Trim/Sulfa Doripenem <2 <0.25 <1 <32 <0.125 I R 3-6 >8 0.5 >1 No interpretive criteria for “I” or “R” MIC (Minimal Inhibitory Concentration) The MIC is the lowest concentration of a drug in mg/ml that inhibits the growth of a microorganism. Read pages 281-284 in the textbook related to “Dilution Methods” for sensitivity testing and Minimum Inhibitory Concentration and answer the questions at the end of this laboratory exercise. Turn the answers in to complete points for this laboratory. MLAB 2434 – Laboratory 12 – Page 5 LABORATORY #12 Antibiotic Susceptibility Testing Kirby-Bauer Standard Antibiotic Sensitivity Zone Sizes Staphylococci Zone Sizes Resistant I Sensitive (S) Resistant I NA ≥ 29 mm ≤ 13 mm 14-16 mm ≥ 17 mm ≤14 mm 15-22 mm ≥23mm ≤14 mm 15-22 mm ≥23mm CPD10 ≤ 17 mm 18-20 mm ≥ 21 mm ≤ 17 mm 18-20 mm ≥ 21 mm Clarithromycin CLR15 < 13mm 14-17 mm > 18mm NA NA NA Ciproflozacin CIP5 ≤ 15 mm 16-20 mm ≥ 21 mm ≤ 15 mm 16-20 mm ≥ 21 mm Erthromycin E15 ≤ 13 mm 14-22 mm ≥ 23 mm NA NA NA Nitrofurantoin F/M300 ≤ 14 mm 15-16 mm ≥ 17 mm ≤ 14 mm 15-16 mm ≥ 17 mm Ofloxacin OFX5 ≤12 mm 13-15 mm ≥16 mm ≤ 12 mm 13-15 mm ≥ 16 mm Tetracycline TE30 ≤14 mm 15-18 mm ≥19 mm ≤ 14 mm 15-18 mm ≥ 19 mm Trimethroprim/ SXT ≤10 mm 11-15 mm ≥16 mm ≤ 10 mm 11-15 mm ≥ 16 mm Antibiotic Symbol Ampicillin AM10 ≤ 28 mm Cefotaxime CTX30 Cefpodoxime Sulfamethoxazole InterMediate (I) Enterobacteriaceae Zone Sizes InterMediate (I) Sensitive (S) *Updated 7/2011 BD BBL Sensi-Disc Antimicrobial Susceptibility Test Discs Insert MLAB 2434 – Laboratory 12 – Page 6 LABORATORY #12 Antibiotic Susceptibility Testing Lab #12: Antibiotic Sensitivity Kirby-Bauer Report Form Points= 10 Name ______ Date Antibiotic Symbol Ampicillin AM10 Cefotaxime CTX30 Cefoxitin FOX30 Cefpodoxime CPD10 Clarithromycin CLR15 Ciproflozacin CIP5 Erythromycin E15 Nitrofurantoin F/M300 Norfloxacin NOR-10 Ofloxacin OFX5 Tetracycline TE30 Trimmethoprim/Sufamethoxazole SXT S. aureus Zone Size R, I or S E. coli Zone Size R, I, or S (mm) (mm) MLAB 2434 – Laboratory 12 – Page 7 LABORATORY #12 Antibiotic Susceptibility Testing Lab # 12:Etest Report Form Points=5 Name ______ Date Organism:______________________ Antibiotic Vancomycin Symbol VA Tolerance limits 0.12-0.5 Levofloxacin LE 0.5-2 Erythromycin EM 0.03-0.25 Trim/Sulfa TS 0.002-32 Doripenem DOR 0.032-0.125 Ceftriaxone TX 0.03-0.12 Reading R,I,S MLAB 2434 – Laboratory 12 – Page 8 LABORATORY #12 Antibiotic Susceptibility Testing Laboratory #12: Antibiotic Susceptibility Testing Study Questions Points= 13 Name Date 1. Describe an E test plastic strip. How are they placed on an agar plate? How do they compare in price to standard antibiotic sensitivity disks? For which types of organisms are the E test especially useful? (2 pts) 2. What is MIC? Briefly explain how an MIC is performed. (2 pts) 3. What is CLSI (http://www.clsi.org) and what part does it play in antibiotic sensitivity testing? (2 pts) 4. When performing microdilution MIC testing, what two control wells are added on each tray and why?(3 pts) MLAB 2434 – Laboratory 12 – Page 9 LABORATORY #12 Antibiotic Susceptibility Testing 5. What is a breakpoint panel? (1 pt) 6. Briefly describe “trailing” and “skipped wells” when performing MICs. How should each be handled? (3 pts.) MLAB 2434 – Laboratory 12 – Page 10