fourth midterm examination
advertisement
USEFUL CONSTANTS
R = 8.31451 J/K-mole = 0.082058 L-atm/K-mole
kB = 1.3087 x 10-23 J/K-molecule
NA = 6.022 x 1023 molecules/mole
h = 6.626 x 10-34 J-s
atomic weights in g/mole: N, 14.0; O, 16.0; D, 2.0
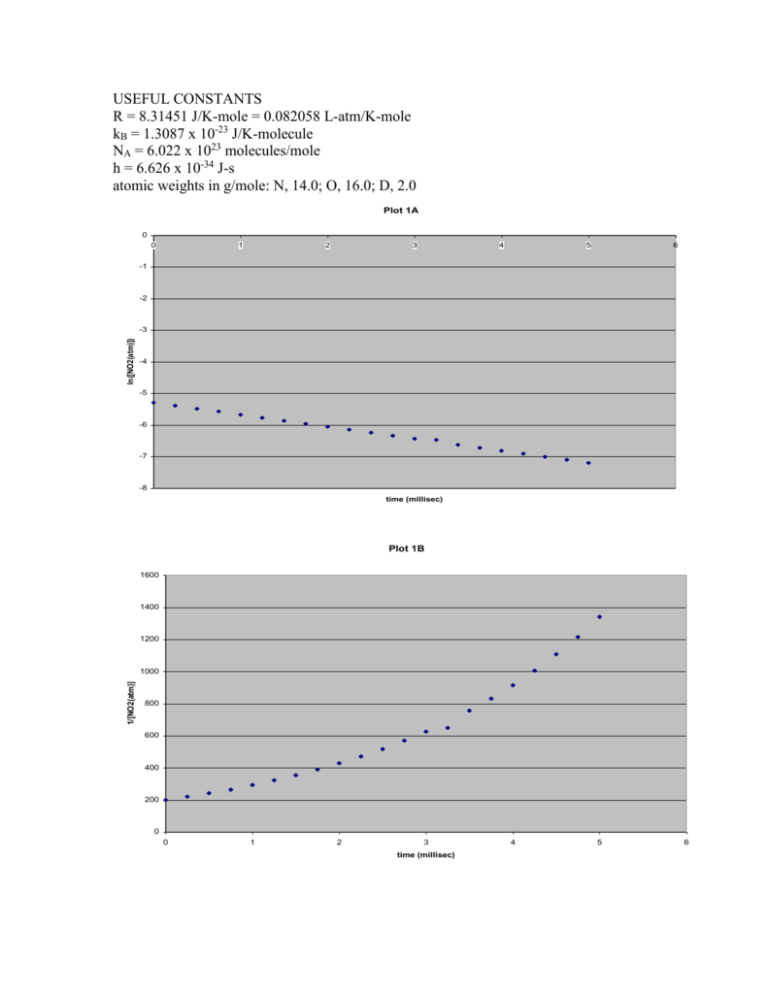
Plot 1A
0
0
1
2
3
4
5
6
-1
-2
ln([NO2(atm)])
-3
-4
-5
-6
-7
-8
time (millisec)
Plot 1B
1600
1400
1200
1/[NO2(atm)]
1000
800
600
400
200
0
0
1
2
3
time (millisec)
4
5
6
NAME:______SOLUTIONS________ Chem 1b, 4th Midterm, Spring, 2005
Important data and constants are provided on the insert. You may use the back for
scratch work but enter all work to be graded in the space provided with each question.
Show your work in all questions involving a calculation in order to receive credit.
1) (40 points) The reaction 2 NO2(g) + O3(g) → N2O5(g) + O2(g) is important in
atmospheric chemistry.
a) In one experiment, the time rate of change of NO2, d[NO2]/dt, was observed to
be -4.0 x 10-4 atm/s. What is the corresponding time rate of change of O2?
From the stoichiometry, rate = (-1/2) d[NO2]/dt = (1/1) d[O2]/dt so
d[O2]/dt = (-1/2)(-4.0 x 10-4 atm/s) = 2.0 x 10-4 atm/s.
b) The figures on the insert show two analyses of data obtained from a single
kinetics experiment at 300 K in which the initial concentrations of O3 and NO2
were 0.200 atm and 0.005 atm, respectively. One plot (1A) shows a plot of
ln([NO2]) versus time. In the other (1B), 1/[NO2] is plotted versus time. A
further series of experiments run at constant [NO2] showed that the initial rate of
the reaction is directly proportional to [O3]. Derive from these results the rate law
for the reaction. Support your answer with an argument.
The non-linearity of the plot of 1/[NO2] versus time rules out second order
dependence on NO2. In contrast, the linearity of the plot of ln([NO2]) versus time
clearly demonstrates first order dependence on NO2, i.e. rate = k(1)[NO2] where
k(1) is a pseudo first order rate constant. The initial rate is directly proportional to
[O3] so k(1) = k[O3]. Therefore, rate = k[O3][NO2].
c) The rate constant for the reaction is 1900 at 300 K. The energy of activation
for the reaction is 29 kJ/mole. Calculate the value of the rate constant at 1000 K.
k = Aexp(-Ea/RT) so kH/kL = [Aexp(-Ea/RTH)]/[Aexp(-Ea/RTL)] =
exp{-(Ea/R)(1/TH - 1/TL)} = exp{-(29000/8.3145)(1/1000 - 1/300)} = exp(8.1348)
k = (1900)exp(8.138) = 6.5 x 106
2) (50 points) The following mechanism has been proposed for the reaction in problem 1:
NO2(g) + O3(g) → NO3(g) + O2(g)
NO3(g) + NO2(g) → N2O5(g)
[step1]
[step 2]
a) List the intermediate(s) in the reaction: NO3
b) Which step is the rate determining step? Provide a brief argument to support
your answer.
The rate is directly proportional to just O3 and NO2, the two species in the first
step so the first step is rate determining. the rate law would be different and more
complicated if the second step were rate determining.
c) What is the sign of the entropy of activation for the forward first step? Why?
Two simple molecules are combined to form a single, more complex species so
the entropy of activation is negative.
d) K1, the equilibrium constant for the first step, can be obtained from
thermodynamic data. Derive a relationship between k-1, the rate constant for the
reverse reaction, and the values of K1 and the forward rate constant, k1.
K1 = k1/k-1 (elementary step!) so k-1 = k1/K1
e) Consider the second step. In which direction, the forward or the reverse, will
the enthalpy of activation be larger? Briefly discuss why. (Hint: NO3 and NO2
are radicals.)
The forward step involves the formation of a bond from very reactive species so
the enthalpy of activation will be very small. The reverse step involves breaking
a bond so the enthalpy of activation will be large.
f) Compare qualitatively at 300 K, the average kinetic energy and average speed
of gaseous N2O5 and NO3.
<KE> = (3/2)RT so <KE> is independent of MW, i.e. same for both.
<KE> = 0.5M<v2> = (3/2)RT so at fixed T, the average speed (specifically the
rms average) is inversely proportional to the square root of the molecular weight.
vrms will be greater for the lighter NO3.
g) What is the average velocity of gaseous NO3 at 300 K?
In the gas phase, translational motion is isotropic so the average velocity is zero.
3) (60 points) The following thermodynamic data at 25.00C are available for
D2O.
species
Hf(kJ/mole)
S(J/K-mole)
D2O(l)
-294.600
75.94
D2O(g)
-249.199
198.339
a) Calculate the vapor pressure of heavy water (D2O) at 25.00C.
Thermodynamics applies to the process D2O(l) D2O(g) and K = pD2O = vp
H = -249.199 - -294.600 = 45.401 kJ = 45,401
S = 198.339 - 75.95 = 122.399 J/K
G =H -TS = 45,401 - (25.00 + 273.15)(122.399) = 8907.7 J
vp = K = exp(-G/RT) = exp{-8907.7/[(8.31451)(298.15)]} = 0.0275 atm
b) The amino acid glycine (molecular weight, 75.0 g/mole) has a very low vapor
pressure. 15.0 g of glycine is dissolved in 100.0 g of D2O. Calculate the vapor
pressure at 25.00C of the resulting solution. List any assumptions that you make
in answering the problem.
If we assume ideal solution behavior, we can use Raoult's Law: pD2O =XD2Op*D2O
nG = (15 g)/(75 g/mole) = 0.20 mole
nW = (100 g)/(20 g/mole) = 5.0 mole
XD2O = (5.0)/(5.0+ 0.20) = 0.962
pD2O = (0.962)(0.0275) = 0.0264 atm
c) A process called lyophilization is used to remove the water from aqueous
solutions and recover the solute, often a very delicate protein. In this process, the
sample is first frozen and then inserted in a vacuum chamber. Describe the
change(s) of state of the water in lyophilization. Why is the sample frozen before
reducing the pressure?
If we quickly lower the pressure when the material is at room temperature (above
the triple point), the liquid water will boil. this rather turbulent process will spray
material throughout the apparatus. If we first freeze the substance, then
vaporization will directly convert the material from the solid to the gaseous state.
Since solids retain their shape, this will not be a turbulent process. Also, the
removal of the water occurs at a lower temperature. This is important if the solute
is very delicate.






