Reactivity of hydrides by in-situ diffraction
advertisement

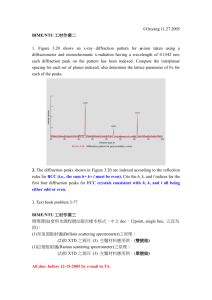
Reactivity of hydrides by in-situ diffraction Y. Filinchuk, I. Dovgaliuk, V. Ban, N. Tumanov, F. Morelle IMCN, Université Catholique de Louvain, pl. L. Pasteur 1, 1348 Louvain-la-Neuve, Belgium In this talk I shall present problems that can be tackled by diffraction experiments under gas pressures. The nature of solid-gas interaction defines what information is assessable: For large-pore systems, macroscopic information can be obtained, such as on occurrence of phase transitions, variation of cell parameters, size of hysteresis loops. These data are very useful in combination with other techniques, phenomenological models and theoretical calculations. For the small-pore systems, in-situ diffraction data allow to access microscopic information, such as location of guest molecules, nature of host-guest interactions and sometimes even energy differences for adsorption sites. When a strong chemical interaction occurs between the crystalline material and the gas, complex sequences of chemical reactions can be disentangled from diffraction data alone. De- and re-hydrogenation reactions of complex (chemical) hydrides are the examples. Typical experimental setups and pressure cells will be presented, as well as our new methodological developments [1] aiming for extracting isosteric heats of adsorption vs loading from diffraction data. Besides the methodological part, I shall focus on solid-state transformations in complex hydrides, induced by variation of temperature and hydrogen pressure. In-situ powder diffraction is our most valuable tool to find new hydrides, understand their reactivity, decomposition pathways, hydrogen release and uptake [2, 3]. Decomposition pathways can be tuned by reaction conditions. All this information inspires us to try different ways to synthesize new hydrides and their decomposition intermediates, for example by using a ball mill, gas atmospheres, ultra-high vacuum etc. At present, the random search for better hydrogen storage systems starts to give the way to the rational design. References [1] Y. Filinchuk, B. Richter, T.R., Jensen V. Dmitriev, D. Chenryshov, H. Hagemann, Angew. Chem. Int. Ed., 50 (2011) 11162-11166. [2] D.B. Ravnsbæk, L.H. Sørensen, Y. Filinchuk, F. Besenbacher, T.R. Jensen, Angew. Chem. Int. Ed., 51 (2012) 3582-3586. [3] R. Černý, Y. Filinchuk, Z. Kristallogr., 226 (2011) 882-891.