Determining the Effects of the Diffusion of Sucrose & Water on
advertisement

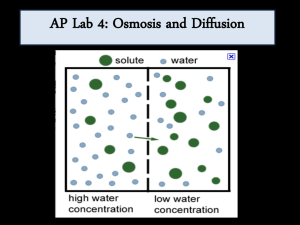
Determining the Effects of the Diffusion of Sucrose & Water on Dialysis Bags Abstract The purpose of this laboratory is to determine if sucrose concentration will affect the diffusion of water into or out of dialysis bags. To determine the affects of molar sucrose concentration, dialysis bags were filled with varying molar sucrose solutions and one with distilled water, massed, and then placed into distilled water for thirty minutes. The percent change in mass was calculated. The dialysis bag with the highest molar concentration of sucrose was predicted to have the greatest percent change in mass and the dialysis bag filled with water was predicted to have little or no percent change in mass. The predictions were supported by the data, where the percent change in mass, of the dialysis bags, per sucrose concentration being 17.6%/M. Or the dialysis bag with 1.0 M sucrose had a change in mass of 36.8% and the water filled dialysis bag had a change in mass of 0.1%. Introduction The purpose of this laboratory is to determine if sucrose concentration will affect the diffusion of water into or out of a dialysis bag. A measurement of concentration is molarity. Molarity is the number of solute molecules (moles) in a given volume (liters) of an aqueous solution, where water is the solvent. As the number of solute molecules increase, in a constant volume, the molarity increases. Since molecules are in constant movement, the more molecules, in a given space, the more they interact. These interactions lead to the diffusion of these molecules. Diffusion is the random movement of molecules from an area of low concentration to an area of high concentration, where osmosis is the diffusion of water molecules through a semi-permeable membrane, as seen in cell membranes. Osmosis is driven by the change in water potential across a cell membrane, where water potential is determined by the amount of available space for solutes. High water potential has more water and less solute and the opposite is true for low water potential. Water molecules diffuse through a semi-permeable membrane from an area of high water potential to an area of low water potential. In cells, molarity can affect the rate at which water and solute molecules move into and out of the cell. Water molecules passing through a cell membrane, is called osmosis, where as solute molecules, such as ions and sugars, passing through the membrane is called dialysis. The rate at which molecules pass through a membrane depends on the change in molarity on either side of the membrane. The larger the change in molarity across the membrane the faster rate of diffusion of molecules will occur. Diffusion of molecules can be observed in cells or in bags made of dialysis tubing. Both cell and dialysis membranes are semi-permeable, meaning that they both allow water to pass, however, they only allow certain solute molecules to pass. If dialysis bags are filled with varying molar sucrose solutions and placed in water then the rate of osmosis may be observed. However, to observe osmosis, the dialysis membranes must be impermeable to sucrose, as is the case for this laboratory. Materials and Methods The end of six, 25 cm strips of dialysis tubing, that were pre-soaked in distilled water, were tied off. Each dialysis tubing was filled with approximately 10 mL of either 0.2 M, 0.4 M, 0.6 M, 0.8 M, or 1.0 M sucrose solution. The sixth dialysis tubing was filled with distilled water to serve as the control. Then the other end of the dialysis tubing was tied off to form dialysis bags. The bags were gently blotted dry and then massed on an electronic balance. The bags were then placed into beakers of approximately 100 mL distilled water for thirty minutes. After thirty minutes the dialysis bags were removed and, again, gently blotted dry and re-massed. The percent change in mass, of each bag, was calculated in order to determine a relative change in mass, thus eliminating the need to have dialysis bags of equal initial mass. The percent change in mass calculation is as follows: final mass (grams) – initial mass (grams) X 100 initial mass (grams) These percent changes in mass for each dialysis bag were then graphed as a function of their molar sucrose solutions (See figure 1.). Results Click here to see the results. Discussion and Conclusion As stated in the introduction, the hypothesis is that if dialysis bags are filled with varying molar sucrose solutions and placed in water then the rate of osmosis may be observed. The rate of osmosis is a function of the change in percent change in mass due to the change in molarity of sucrose. This hypothesis can be supported by the data collected. Each dialysis bag was filled with a different molar sucrose solution, starting from 0.2 M and ending with 1.0 M. The sixth bag was filled with distilled water to show that there would not be any change in mass, due to osmosis. Figure 1 shows a linear increase, in the percent change in mass per Molarity of sucrose, of 17.6%/M. As expected, the dialysis bag filled with water show little to no percent change in mass being 0.1%, where as the remaining dialysis bags showed an increase in percent change in mass. In comparison to each other, the dialysis bag filled with 0.2 M sucrose had the least percent change in mass of 7.4%, where as the bag filled with 1.0 M sucrose had the highest percent change in mass of 37.2%. The linear increase in the percent change in mass as a function of sucrose molarity shows that water diffused from an area of relative high water concentration, or distilled water outside the bags, to an area of lower water concentration, or high sucrose molarity inside the bags. Bibliography Advanced Placement Program, AP Biology Lab Manual for Students, Laboratory One Diffusion & Osmosis, pp. 1-18. The College Board. 2001 Mr. Adame’s Lecture on Diffusion & Osmosis, September 12th, 2005. Campbell & Reese, Biology. Chapter 4 & 8. Benjamin-Cummings, 2003.