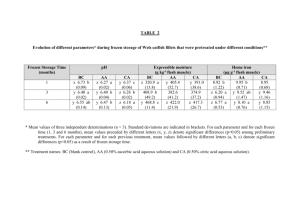
Investigation rancidity - digital
advertisement

An investigation of rancidity inhibition during frozen storage of Wels catfish (Silurus glanis) fillets by previous ascorbic and citric acid treatment Parastoo Pourashouria, Bahare Shabanpoura , Santiago P. Aubourgb,*, Javad Daghigh Rohic & Ali Shabania a Department of Fishery. Gorgan University of Agricultural Sciences & Natural Resources, Gorgan (Iran) b Department of Food Technology. Instituto de Investigaciones Marinas (CSIC), Vigo (Spain) c Inland Waters Aquaculture Institute, Bandar Anzali (Iran) * Correspondent: Fax: + 34 986292762; saubourg@iim.csic.es SUMMARY The effect of preliminary ascorbic and citric acid (AA and CA, respectively) soaking treatments on the rancidity development in Wels catfish (Silurus glanis) fillets during further frozen storage (-18ºC; up to six months) was studied. Rancidity development was measured by biochemical quality indices (formation of free fatty acids, peroxides and secondary oxidation products) and compared to sensory analysis (appearance, rancid odour and consistency) and general chemical analyses (pH, expressible moisture and heme iron contents). When compared to control samples, AA and CA treated samples showed a lower (p<0.05) formation of primary and secondary lipid oxidation compounds that was corroborated by a longer (p<0.05) shelf-life time as a result of a lower rancid odour formation. According to these results, control samples showed a higher (p<0.05) heme iron breakdown (months 3-6) and a lower (p<0.05) water holding capacity (higher expressible moisture value) (month 6). Keywords: Wels catfish, fillet, ascorbic acid, citric acid, frozen storage, rancidity Running Head: Rancidity inhibition in frozen Wels catfish 2 INTRODUCTION Aquatic species are known to provide high contents of important constituents for the human diet such as nutritional and digestive proteins, lipid-soluble vitamins (A and D, mainly), microelements (I, F, Ca, Cu, Zn, Fe and others) and polyunsaturated fatty acids (PUFA) (Piclet, 1987; Simopoulos, 1997). However, marine and fresh water products are known to easily deteriorate during processing and storage due to the action of different factors as microbiological development, endogenous enzyme activity, non-enzymatic lipid oxidation and browning (Cheftel & Cheftel, 1976). Fish lipid degradation is now the subject of a great deal of attention. Thus, PUFA oxidation can lead to the formation of primary and secondary lipid oxidation products, which can finally contribute to loss of essential nutrients and detrimental sensory quality (Hsieh & Kinsella, 1989; Harris & Tall, 1994). In order to minimise such undesirable effects, different technological possibilities have been outlined such as low temperature storage, appropriate packaging, glazing including protecting chemicals and the incorporation of antioxidants (Toledo-Flores & Zall, 1992; Richards et al., 1998; Lin & Lin, 2005). Concerning antioxidant employment, recent efforts are focused on the replacement of synthetic antioxidants by natural ones, which may provide nutritional and therapeutic effects (Frankel, 1995; Decker, 1998). In this sense, ascorbic and citric acids (AA and CA, respectively) and their salts are widely known for their role as chelators, acidulants in biological systems and synergists of primary antioxidants, so that a profitable effect on fish oil and emulsions (Kelleher et al., 1992; Osborn-Barnes & Akoh, 2003), minced fish (Hwang & Regenstein, 1988; Stodolnik et al., 1992) and fish fillets (Badii & Howell, 2002; Aubourg et al., 2004) have been observed. 3 Wels catfish (Silurus glanis) is an Eurasian fresh water fish supporting an important commercial interest (FAO, 2006). Previous technological research on this species accounts for its quality assessment as a result of stunning and slaughter conditions (Marx et al., 1999), ice storage (Manthey et al., 1988) and freeze-thaw cycles (Benjakul & Bauer, 2001). Analysis of muscle constituents (Draisci et al., 1999; Mendil & Uluozlu, 2007) and volatile compounds from cooked fillets (Hallier et al., 2005) has also been carried out. The present work focuses on Wels catfish trading as a frozen product. In it, the effect of a previous AA and CA soaking on lipid stability during further fillet frozen storage was investigated. MATERIALS AND METHODS Raw fish, sampling, processing and chemicals Fresh Wels catfish (Silurus glanis) (36 individual fishes) were captured in January 2006 from Anzali Lagoon (Bandar Anzali, Iran). The weight of the specimens ranged between 1500g and 1700g. The fish were carefully gutted, dressed and filleted by hand (North Fillet manufactory; Bandar Anzali, Iran). The weight of each fillet ranged between 570g and 700g. Fillets (from 27 individual fishes) were then immersed either in water (Blank Control; BC treatment), 0.50% AA aqueous solution (AA treatment) or 0.50% CA aqueous solution (CA treatment) in an isothermal room at 4ºC. After five minutes, fillets from all solutions were removed, packaged in individual polyethylene bags and placed in a freezer at –40˚C. Antioxidant concentration and dipping time were chosen according to previous related research (Chapman et al., 1993; Aubourg et al., 2004). After 24 h at –40˚C, all fish fillets were placed in a freezer at –18˚C. Sampling was undertaken at 1, 3 and 6 months of frozen storage at –18ºC and on the raw fish (initial material; 9 individual fishes). 4 Experiments were performed at Inland Waters Aquaculture Institute (Bandar Anzali, Iran). For each treatment (BC, AA and CA), three different fish batches (n = 3) were considered and studied separately to achieve the statistical study. Chemicals (solvents and reactants) employed through the study were reagent grade (E. Merck, Darmstadt, Germany). Sensory analysis Sensory analyses were conducted by a taste panel consisting of 5-7 experienced judges, according to the guidelines presented in Table 1 (DOCE, 1989). Four categories were ranked: highest quality (E), good quality (A), fair quality (B) and rejectable quality (C). Sensory assessment of the fish fillet included the following parameters: Flesh appearance, rancid odour and flesh consistency. At each sampling time, the different fish fillets were thawed and then analysed in the same session. The fish fillets were served to the panel members in the individual polyethylene bags where they had been kept frozen and were scored individually. General chemical analyses Moisture content was determined by weight difference between the fresh homogenised muscle (1-2 g) and the muscle after 24 h at 105˚C. Results were calculated as g water kg-1 flesh muscle. Lipids were extracted according to the Bligh & Dyer (1959) method. Lipid content is expressed as g kg-1 flesh muscle. Evolution of the pH value was carried out according to the Scott et al. (1988) method. For it, five grams of Wels catfish mince were homogenised for 1 min with 45 ml distilled water and then measured in a standardised portable digital pH meter (Beckman Ф40, Krefeld, Germany). 5 Expressible moisture was determined according to the Suvanich et al. (2000) method. The drip was calculated as g kg-1 flesh muscle. Heme iron was determined by employing the acidified acetone extraction method developed by Clark et al. (1997). In it, the concentration of total pigments (TP) in fish muscle (μg hematin g-1 flesh muscle) was obtained as follows: TP = A x 6800 / w, where A is the absorbance reading at 640 nm and w is the sample weight (g). Then, the heme iron content was calculated with the factor of 0.0882 μg heme iron μg-1 hematin (Merck Index, 1989). Results are expressed as μg heme iron g-1 flesh muscle. Lipid damage measurements Free fatty acid (FFA) content was determined in the lipid extract by the Egan et al. (1997) method. Results are expressed as g oleic fatty acid kg-1 lipids. Peroxide value (PV) was determined in the lipid extract according to the method described by Egan et al. (1997). Results are expressed as meq oxygen kg-1 lipids. The thiobarbituric acid index (TBA-i) (mg malondialdehyde kg-1 flesh muscle) was determined in a 5% trichloracetic acid extract according to the Kirk & Sawyer (1991) method. Statistical analysis Data from the different quality parameters were subjected to the one-way ANOVA method (p<0.05) (Statsoft, 1994). Comparison of means was performed using a least-squares difference (LSD) method. 6 RESULTS AND DISCUSSION Evolution of general chemical parameters Moisture contents ranged between 800 and 830 g kg-1 flesh muscle in all samples. Lipid contents ranged between 22.0 and 40.8 g kg-1 flesh muscle. Variations found in both constituent (water and lipids) contents did not show a definite effect of the frozen storage or antioxidant treatment and may be explained as a result of individual fish-to-fish variation (Aubourg et al., 2002; Aubourg et al., 2004). In this sense, lipid content in fish species has shown wide variations as a result of endogenous and exogenous effects (Cheftel & Cheftel, 1976; Pearson et al., 1977); in addition, an inverse ratio between water and lipid contents has been shown in fish species (Piclet, 1987). At month 1, samples corresponding to both previous AA and CA treatments showed a lower pH value than their corresponding control samples (Table 2). This lower value was maintained during the 3-6 month period for CA samples when compared to control and AA treated fish; no differences were observed in the 3-6 month period between BC and AA samples. A pH rising during freezing and frozen storage was not observed for BC samples (initial pH fish value: 6.91±0.34), according to previous research where frozen storage did not lead to pH differences between fresh muscle and the different frozen storage times (Eun et al., 1994; Hurling & McArthur, 1996). Expressible moisture (Table 2) showed a progressive increase with storage time in control samples as a result of a marked lowering of the water holding capacity (WHC). However, in the case of AA and CA treated samples, no differences in WHC were obtained throughout the storage time. When comparing the different treatments, a higher expressible moisture content for AA and CA treated samples was observed at month 1 than in the control samples; this result may be explained as a result of the previous acid treatment, according to some related 7 research concerning malic acid pretreatment (Chen et al., 1998). However, at month 6, when fish damage is supposed to be increased in all kinds of samples, a higher expressible moisture content is obtained for BC samples than in their counterpart acid treated fishes. During the frozen storage of fish, lipid oxidation has shown to enhance protein denaturation and detrimental texture changes (Mackie, 1993; Saeed & Howell, 2002). In such storage conditions, one consequence of protein denaturation has been reported to be the reduction of WHC of the fish muscle (Simeonidou et al., 1997; Suvanich et al., 2000). Since a higher WHC was obtained in the present research for the acid treated fish, a lower protein denaturation and lipid oxidation compound formation can be inferred as a result of the antioxidant preliminary treatment. Heme iron content (Table 2) was found lower in control samples than in both acid treated fish when considering a frozen storage of 3 and 6 months. A slight decrease in heme iron content was observed with storage time for AA treated samples, while no differences throughout the experiment could be outlined for CA treated fish; however, control fish showed a strong heme iron breakdown at month 3, followed by no change at the end of the experiment. As a result, a marked effect of previous antioxidant treatment has been proved on heme iron retention in frozen fish. Previous research has shown that food processing (Turhan et al., 2004; Chaijan et al., 2005) and chemical treatment (Schricker & Miller, 1983) may lead to degradation of the heme iron into nonheme iron (free form). Since nonheme iron has been reported to be one of the major catalysts of lipid oxidation (Hsieh & Kinsella, 1989; Huang et al., 1993), a lower muscle content of this metabolite would allow a higher rancidity stability and accordingly, a larger shelf-life time of the corresponding fish product. From a nutritional point of view, a great advantage is also accorded to the heme iron content in front of its counterpart free form; thus, nonheme iron has shown a lower bioavailability than heme one (Monsen & Balintfy, 1982). 8 Lipid hydrolysis development A significant (p<0.05) hydrolysis development was observed for the three kinds of treatments during the frozen storage (Figure 1), according to previous research on other frozen fish species (Aubourg et al., 2002; Aubourg et al., 2005). Thus, control samples showed a sharp increase (p<0.05) at month 3 and then remained relatively constant till the end of the experiment, while AA and CA treated samples showed a progressive increase (p<0.05) throughout the whole experiment. Comparison among treatments did not provide a definite pattern. Thus, a higher (p<005) hydrolysis development at month 1 was observed for CA soaked samples, while at month 3 BC treatment led to the highest (p<0.05) values; finally, no significant differences (p>0.05) could be outlined at the end of the experiment. The formation of FFA itself does not lead to nutritional losses. However, examining the extent of lipid hydrolysis was deemed important to the study because free fatty acids (FFA) are known to undergo further oxidation to produce low molecular weight compounds that are responsible for the rancid off-flavour and taste of fish and fish products (Vidya Sagar Reddy & Srikar, 1996; Refsgaard et al., 2000) and have great influence on protein denaturation (Mackie, 1993; Sikorski & Kolakowska, 1994). Lipid oxidation development Lipid oxidation development was measured according to the peroxide formation (primary oxidation compounds) and the thiobarbituric acid index (secondary oxidation compounds). A strong (p<0.05) peroxide formation (Figure 2) for control samples could be outlined throughout the experiment, so that a PV > 20 was obtained at the end of the experiment. However, AA and CA treated samples showed a progressive but slow increase (p<0.05) with frozen time, so that values above 10 were not attained even at the end of the storage time. Comparison among the different kinds of treatments led to a higher (p<0.05) primary oxidation 9 development at month 6 for BC samples, while no differences (p>0.05) were detected between AA and CA treated samples throughout the whole experiment. The TBA-i assessment (Figure 3) led to similar results than in the case of the above mentioned peroxide analysis (Figure 2). Thus, a gradual increase (p<0.05) during frozen storage for all samples was observed that was markedly higher in the case of control samples. Comparison among treatments revealed the AA < CA < BC TBA-i increasing (p<0.05) sequence at the end of the storage. However, no significant (p>0.05) differences could be assessed at months 1 and 3 among the different kinds of samples. Sensory analysis Progressive score decreases with the frozen storage time were observed for the three attributes considered in all kinds of samples (Table 3) according to previous results on fish species under similar conditions (Aubourg et al., 2002; Aubourg et al., 2005). Comparison among treatments showed no differences when considering the flesh consistency. However, when the rancid odour development is evaluated, a lower score is obtained at month six for the BC samples than in both acid treated samples; in such case, control samples were not acceptable, so that this attribute showed to be limiting when considering the shelf-life time in the present experiment. Further, odour assessment showed a better score at month 3 for AA treated samples than for their corresponding BC and CA ones. Among the different kinds of molecules produced as a result of lipid oxidation, secondary ones are considered the chief compounds responsible for oxidised flavours (Kurade & Baranowski, 1987; White, 1994). Accordingly, a close relationship between the rancid odour development and the TBA-i assessment has been obtained in the present experiment. Finally, flesh appearance analysis led to a better score at month 1 for CA treated fish, although no differences in the 3-6 month period were obtained among the three kinds of samples. 10 CONCLUDING REMARKS In the present research, previous AA and CA soaking treatments have led to an increased rancidity stability of frozen Wels catfish fillets. Thus, a lower (p<0.05) primary and secondary lipid oxidation compound formation was obtained, being this result in agreement with a lower heme iron breakdown into non heme iron. According to these results, a lower rancid odour development was obtained from the sensory acceptance results, so that a longer shelf-life time was accorded to acid treated fish. At the same time, a higher WHC was observed for acid treated fish as a result of a lower protein denaturation, closely related to a lower lipid oxidation development. Both acids offer many advantages for their employment as antioxidant molecules during fish processing such as easy availability, high water solubility, low commercial value and a high level allocated for their use. Consequently, the employment of both acids is firmly recommended as a previous treatment to the chilled and frozen storages, alone or in combination with other protective strategies such as glazing, modified atmosphere and vacuum packaging, and so on. Acknowledgments The authors appreciate the Inland Waters Aquaculture Institute (Bandar Anzali, Iran), and North Fillet manufactory (Bandar Anzali, Iran) for experimental and technical assistances. 11 FIGURE LEGENDS Figure 1: Free fatty acid (FFA; g oleic acid kg-1 lipids) formation* during frozen storage of Wels catfish fillets that were pretreated under different conditions** * Mean values (n = 3) of three independent determinations. Standard deviations are indicated by bars. Initial fish FFA value: 9.3 ± 2.5. ** Treatment names as expressed in Table 2. Figure 2: Peroxide value (PV; meq active oxygen kg-1 lipids) evolution* during frozen storage of Wels catfish fillets that were pretreated under different conditions** * Mean values (n = 3) of three independent determinations. Standard deviations are indicated by bars. Initial fish PV: 3.6 ± 0.9. ** Treatment names as expressed in Table 2. Figure 3: Thiobarbituric acid index (TBA-i; mg malondialdehyde kg-1 flesh muscle) assessment* during frozen storage of Wels catfish fillets that were pretreated under different conditions** * Mean values (n = 3) of three independent determinations. Standard deviations are indicated by bars. Initial fish TBA-i: 0.46 ± 0.04. ** Treatment names as expressed in Table 2. 12 REFERENCES Aubourg, S., Lehmann, I. & Gallardo, J. (2002). Effect of previous chilled storage on rancidity development in frozen horse mackerel (Trachurus trachurus). Journal of the Science of Food and Agriculture, 82, 1764-1771. Aubourg, S., Pérez-Alonso, F. & Gallardo, J. (2004). Studies on rancidity inhibition in frozen horse mackerel (Trachurus trachurus) by citric acid and ascorbic acids. European Journal of Lipid Science and Technology, 106, 232-240. Aubourg, S., Rodríguez, A. & Gallardo, J. (2005). Rancidity development during frozen storage of mackerel (Scomber scombrus): Effect of catching season and commercial presentation. European Journal of Lipid Science and Technology, 107, 316-323. Badii, F. & Howell, N. (2002). Effect of antioxidants, citrate, and cryoprotectants on protein denaturation and texture of frozen cod (Gadus morhua). Journal of the Agricultural and Food Chemistry, 50, 2053-2061. Benjakul, S. & Bauer, F. (2001). Biochemical and physicochemical changes in catfish (Silurus glanis Linne) muscle as influenced by different freeze-thaw cycles. Food Chemistry, 72, 207-217. Bligh, E. & Dyer, W. (1959). A rapid method of total extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911-917. Chaijan, M., Benjakul, S., Visessanguan, W. & Faustman, C. (2005). Changes of pigments and colour in sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) muscle during iced storage. Food Chemistry, 93, 607-617. Chapman, K., Sagi, I., Hwang, K. & Regenstein, J. (1993). Extra-cold storage of hake and mackerel fillets and mince. Journal of Food Science, 58, 1208-1211. 13 Cheftel, J. & Cheftel, H. (1976). Introducción a la Bioquímica y Tecnología de Alimentos. Pp. 237-323. Zaragoza, Spain: Editorial Acribia. Chen, H., Chow, C. & Ochiai, Y. (1998). Effects of acid and alkaline reagents on the color and gel-forming ability of milkfish kamaboko. Fisheries Science, 64, 160-163. Clark, E., Mahoney, A. & Carpenter, C. (1997). Heme iron and total iron in ready-to-eat chicken. Journal of the Agricultural and Food Chemistry, 45, 124-126. Decker, E. (1998). Strategies for manipulating the prooxidative/antioxidative balance of foods to maximize oxidative stability. Trends in Food Science and Technology, 9, 241-248. DOCE (1989). Baremo de Clasificación de Frescura. In: Diario Oficial de las Comunidades Europeas (L5/21, 07.01.1989). Pp. 5-6. Brussels, Belgium: European Commission. Draisci, R., Marchiafava, C., Palleschi, L., Ferretti, E., Anastasio, A. & Visciano, P. (1999). Determination of muscle compounds in freshwater fish samples collected in Italy. Industrie Alimentari, 38, 374-376. Egan, H., Kirk, R. & Sawyer, R. (1997). Pearson's Chemical Analysis of Foods. 9th Edition. Pp. 609-634. Edinburgh (Scotland, UK): Churchill Livingstone. Eun, J., Boyle, J. & Hearnsberger, J. (1994). Lipid peroxidation and chemical changes in catfish (Ictalurus punctatus) muscle microsomes during frozen storage. Journal of Food Science, 59, 251-255. FAO (2006). Fishery statistics. Capture production. In: Food and Agriculture Organization of the United Nations. Vol. 98/1. P. 94. Rome (Italy): Yearbook 2004. Frankel, E. (1995). Natural and biological antioxidants in foods and biological systems. Their mechanism of action, applications and implications. Lipid Technology, July, 77-80. Hallier, A., Prost, C. & Sérot, T. (2005). Influence of rearing conditions on the volatile compounds of cooked filets of Silurus glanis (European catfish). Journal of the Agricultural and Food Chemistry, 53, 7204-7211. 14 Harris, P. & Tall, J. (1994). Substrate specificity of mackerel flesh lipopolygenase. Journal of Food Science, 59, 504-506, 516. Hsieh, R. & Kinsella, J. (1989). Oxidation of polyunsaturated fatty acids: mechanisms, products, and inhibition with emphasis on fish. Advances in Food Research and Nutrition, 33, 233-341. Huang, C.-H., Hultin, H. & Jafar, S. (1993). Some aspects of Fe2+-catalyzed oxidation of fish sarcoplasmic reticular lipid. Journal of Agricultural and Food Chemistry, 41, 18861892. Hurling, R. & McArthur, H. (1996). Thawing, refreezing and frozen storage effects on muscle functionality and sensory attributes of frozen cod (Gadus morhua). Journal of Food Science, 61, 1289-1296. Hwang, K. & Regenstein, J. (1988). Protection of menhaden mince lipids from rancidity during frozen storage. Journal of Food Science, 45, 1120-1124. Kelleher, S., Silva, L., Hultin, H. & Wilhelm, K. (1992). Inhibition of lipid oxidation during processing of washed, minced Atlantic mackerel. Journal of Food Science, 57, 11031108. Kirk, R. & Sawyer, R. (1991). Pearson's Composition and Analysis of Foods. 9th Edition. Pp. 642-643. Singapore (Singapore): Longman Scientific and Technical. Kurade, S. & Baranowski, J. (1987). Prediction of shelf-life of frozen minced fish in terms of oxidative rancidity as measured by TBARS number. Journal of Food Science, 52, 300302, 311. Lin, C. & Lin, C. (2005). Enhancement of the storage quality of frozen bonito fillets by glazing with tea extracts. Food Control, 16, 169-175. Mackie, I. (1993). The effect of freezing on flesh proteins. Food Reviews International, 9, 575610. 15 Manthey, M., Karnop, G. & Rehbein, H. (1988). Quality changes of European catfish (Silurus glanis) from warm-water aquaculture during storage on ice. International Journal of Food Science and Technology, 23, 1-9. Marx, H., Sengmueller-Sieber, T., Hoffmann, R. & Stolle, A. (1999). Stress and product quality of trout, catfish and flounder at stunning and slaughter. Archiven für Lebensmittelhygiene, 50, 37-40. Mendil, D. & Uluozlu, O. (2007). Determination of trace metal levels in sediment and five fish species from lakes in Tokat, Turkey. Food Chemistry, 101, 739-745. Merck Index (1989) An encyclopaedia of chemicals, drugs, and biologicals, 11th ed., Rahway, NJ (USA): Merck and Company. Monsen, E. & Balintfy, J. (1982). Journal of the American Dietetic Association, 80, 307-311. Osborn-Barnes, H. & Akoh, C. (2003). Copper-catalyzed oxidation of a structured lipid-based emulsion containing α-tocopherol and citric acid: Influence of pH and NaCl. Journal of the Agricultural and Food Chemistry, 51, 6851-6855. Pearson, A., Love, J. & Shorland, F. (1977). “Warmed-over” flavor in meat, poultry and fish. Advances in Food Research, 23, 2-61. Piclet, G. (1987). Le poisson aliment. Composition – intérêt nutritionnel. Cahiers de Nutrition et Diététique, XXII, 317-335. Refsgaard, H., Brockhoff, P. & Jensen, B. (2000). Free polyunsaturated fatty acids cause taste deterioration of salmon during frozen storage. Journal of the Agricultural and Food Chemistry, 48, 3280-3285. Richards, M., Kelleher, S. & Hultin, H. (1998). Effect of washing with or without antioxidants on quality retention of mackerel fillets during refrigerated and frozen storage. Journal of the Agricultural and Food Chemistry, 46, 4363-4371. 16 Saeed, S. & Howell, N. (2002). Effect of lipid oxidation and frozen storage on muscle proteins of Atlantic mackerel (Scomber scombrus). Journal of the Science of Food and Agriculture, 82, 579-586. Schricker, B. & Miller, D. (1983). Effects of cooking and chemical treatment on heme and nonheme iron meat. Journal of Food Science, 48, 1340-1349. Scott, D., Porter, R., Kudo, G., Miller, R. & Koury, B. (1988). Effect of freezing and frozen storage of Alaska pollack on the chemical and gel-forming properties of surimi. Journal of Food Science, 50, 723-726. Sikorski, Z. & Kolakowska, A. (1994). Changes in protein in frozen stored fish. In: Seafood proteins (edited by Z. Sikorski, B. Sun Pan & F. Shahidi). Pp. 99-112. New York (USA): Chapman and Hall. Simeonidou, S., Govaris, A. & Vareltzis, K. (1997). Effect of frozen storage on the quality of whole fish and fillets of horse mackerel (Trachurus trachurus) and Mediterranean hake (Merluccius mediterraneus). Zeitschrift für Lebensmittel Untersuchung und Forschung, 204, 405-410. Simopoulos, A. (1997). Nutritional aspects of fish. In: Seafood from producer to consumer, Integrated approach to quality (edited by J. Luten, T. Börrensen & J. Oehlenschläger). Pp. 589-607. London (UK): Elsevier Science. Statsoft. (1994). Statistica for Macintosh. Statsoft and its licensors. Tulsa, Ok (USA): Statsoft. Stodolnik, L., Blasiak, E. & Broszedzka, H. (1992). Effect of Tween 80, citric acid and acetylsalicylic acids on changes in muscle tissue lipids of Baltic herrings during storage. Chlodnictwo, 27, 29-35. Suvanich, V., Jahncke, M. & Marshall, D. (2000). Changes in selected chemical quality characteristics of channel catfish frame mince during chill and frozen storage. Journal of Food Science, 65, 24-29. 17 Toledo-Flores, L. & Zall, R. (1992). Methods for extending the storage life of fresh tropical fish. In: Advances in Seafood Biochemistry (edited by G. Flick & R. Martin). Pp. 233243. Lancaster, PA (USA): Technomic Publishing. Turhan, S., Ustun, N. & Altunkaynak, T. (2004). Effect of cooking methods on total and heme iron contents of anchovy (Engraulis encrasicholus). Food Chemistry, 88, 169-172. Vidya Sagar Reddy, G. & Srikar, L. (1996). Effect of preprocess ice storage on lipid change of Japanese threadfin bream (Nemipterus japonicus) mince during frozen storage. Asian Fisheries Science, 9, 109-114. White, P. (1994). Conjugated diene, anisidine value and carbonyl value analyses. In: Methods to assess quality and stability of oils and fat-containing foods (edited by K. Warner & M. Eskin). Pp. 159-178. Champaign, Illinois (USA): AOCS Press. 18 TABLE 1 Scale employed for evaluating the sensory quality of frozen Wels catfish fillets* Attribute Flesh Appearance Rancid Odour Flesh Consistency E A B C (Highest quality) (Good quality) (Fair quality) (Rejectable quality) Strongly Still hydrated and Slightly dry and Yellowish and hydrated and pink; myotomes pale; myotomes dry; myotomes pink; myotomes adhered adhered in groups totally separated totally adhered Sharp seaweed Weak seaweed Slightly sour and Sharply sour and and shellfish and shellfish incipient rancid rancidity Presence or Firm and elastic; Presence of Important shape partial pressure signs mechanical signs; changes due to disappearance of disappear elasticity notably mechanical rigor mortis immediately and reduced factors symptoms completely * Adapted from DOCE (1989). 19