2 - OSU Chemistry
advertisement

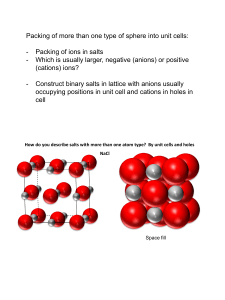
Eutactic Structures (a) Octahedral and Tetrahedral Holes Ionic structures have traditionally been described by starting with a close packing of anions and inserting cations in the voids present in the close packed anionic system. Although this is useful for visualization purposes a literal acceptance of this doctrine has several flaws, two of which I point out below: (1) The Coulombic interaction between anions is repulsive, so they would prefer to maximize their separation rather than minimize it. (2) This assumes that anions are much larger than cations, but modern techniques have shown that in crystals this is not necessarily true (i.e. O2- has a crystal radius of 126 pm, while Na+ has a crystal radius of 116 pm). The arrangements of ions in crystals have been investigated by Mike O'Keeffe (Acta Cryst. A 33, 924-927 (1977)) who concludes that the ions in ionic structures approximate close packing arrangements in order to maximize the volume of the structure, while keeping the cation-anion distance at its optimal value. He calls these structures Eutactic. Keeping these considerations in mind it is still a useful visualization tool to consider ionic structures beginning from the closest packing concept. The figure below shows the location of the octahedral (O) and tetrahedral holes (T and T') in relation to the close packed layers. When you look at the image perpendicular to the layers you see the octahedron looking down a threefold axis (a triangular face of the octahedron is in the plane of the screen), the tetrahedra are also seen looking down a threefold axis (looking down one of the T-O bonds). The T-O bond perpendicular to the plane of the screen is pointing up for the T holes and down for the T' holes. The lower figure shows the location of the octahedral holes in the FCC cell (which results from cubic close packing). There is an octahedral hole at the midpoint of each edge, and in the center of the unit cell, where the octahedron can clearly be seen. The tetrahedral holes are located in the center of each octant of the cubic cell. Remembering that atoms located on a corner, edge or face are only 1/8, 1/4 and 1/2 contained in the unit cell, we can count the number of close packing ions and holes per FCC unit cell. 4 Close packing ions 4 Octahedral holes 8 Tetrahedral holes For an HCP unit cell the ratios are the same, but there are only half as many ions per unit cell. (b) Structures Based on Filling of Octahedral Holes There are several structures that can be formed by filling some or all of the octahedral holes in a close paced (eutactic) anion lattice. The exact structure type depends upon the fraction of octahedral holes that are filled and whether the anion lattice is hcp or ccp. Structure Type Rock Salt (NaCl) NiAs CdCl2 CdI2 TiO2 (rutile) Al2O3 (corundum) Fraction of Octahedral Holes Filled 100% 100% 50% 50% 50% 66.7% Close Packed Arrangement Cubic cp Hexagonal cp Cubic cp Hexagonal cp Hexagonal cp* Hexagonal cp Space Group Fm3m P63/mmc P3m1 P42/mnm R3c *The hcp layers in rutile are buckled, so it is an approximation to call them close packed. Pictures and crystallographic descriptions of each of these structures can be found by clicking on the name of the structure in the table above. A few comparisons between these structures are given below. 1. All of the octahedral holes are filled in both the rock salt and NiAs structures, but the contrast in anion lattice packing (ccp vs. hcp) has some important consequences. If we view each structure as being constructed from cation centered polyhedra, each octahedron in rock salt shares edges with its neighbors, while in NiAs the Ni-centered octahedron shares both edges and faces with neighboring octahedra. This results in a fairly short cation-cation distance in NiAs, which makes this structure type unstable when the cation-anion bond becomes too ionic. 2. Another difference between rock salt and NiAs is the anion coordination. In rock salt it is octahedral, which means that both cation and anion have the exact same coordination environment. In NiAs both ions are six-coordinate but the anion has trigonal prismatic coordination. The higher symmetry of the rock salt structure is very favorable for ionic compounds (though the rock salt structure is not limited to ionic compounds), provided there is a 1:1 stoichiometry. 3. Each octahedron in corundum shares both edges and faces, as in the NiAs structure. However, the vacancies on the octahedral holes are distributed so that each octahedron shares only one of its faces with a neighboring octahedron, as opposed to sharing two opposite faces as in NiAs. This modification allows the cations in corundum to shift away from each other, thereby reducing the cation-cation repulsion. This helps to explain why ionic compounds, such as Al2O3 and Ga2O3, are found with the corundum structure. 4. From the table above it’s not clear what distinguishes the rutile structure from the CdI2 structure. Both can be described as hcp arrays of anions (noting that the anion array is distorted in rutile) with 50% of the octahedral holes filled. However, they differ in the way the octahedral holes are filled. In rutile the octahedral holes are filled in a manner that produces chains of edge-sharing octahedra running parallel to thee c-axis. These chains are linked with other chains corners to form a 3D structure. In CdI2 layers of filled octahedral holes alternate with layers of vacant octahedral holes to form sheets of edgesharing octahedral which are held together by relatively weak van der Waals interactions between iodine layers. This type of interaction is most stable when the anion is relatively large and has a low formal charge (soft anions such as I- and Br-). There are several hydroxides that take the CdI2 structure. In these compounds the hydrogen ions reside between the oxide layers and hydrogen bonding is responsible for holding the layers together. 5. The CdCl2 structure is very similar to CdI2, with van der Waals interactions holding layers of edge shared octahedra together. They differ primarily in their anion packing arrangement: ccp in CdCl2 and hcp in CdI2. Both structure types can be considered layered 2D structures in terms of their bonding and should be amenable to intercalation of cations in between the layers. (c) Structures Based on Filling of Tetrahedral Holes The following structure types can be formed by filling tetrahedral holes of a close packed array of anions (usually) Structure Type (Anti)fluorite Wurtzite (zincite) Sphalerite (zinc blende) Diamond Fraction of Tetrahedral Holes Filled 100% 50% Close Packed Arrangement Space Group Cubic cp Hexagonal cp Fm3m P63mc 50% Cubic cp P43m 50% Cubic cp Fd3m A few notes on structures based on filling tetrahedral holes are given below: 1. Just as filling all of the octahedral holes in a ccp anion array leads to the rock salt structure where each octahedron shares all of its edges with neighboring octahedra, filling all of the tetrahedral holes in a ccp array leads to the antifluorite structure where each tetrahedron shares each of its edges with neighboring tetrahedra. A similar situation exists for an hcp anion array where face sharing polyhedra results (NiAs face sharing octahedra). However, as far as I know, face sharing tetrahedra do not exist (you can geometrically show that the M- M distance is shorter than the M-X distance!). Consequently, it is not possible to fill all of the tetrahedral holes in an hcp anion array. 2. The fluorite (e.g. CaF2) and antifluorite (Na2O) differ from each other in that the anion and cation positions are reversed. In the fluorite structure the cation is surrounded by 8 anions in a cubic coordination, while the anion is tetrahedrally coordinated (and vice versa in antifluorite). 3. The sphalerite and wurtzite structures have the common feature that both anions and cations are tetrahedrally coordinated. Furthermore, in each structure the tetrahedra are linked to each other by corner sharing. To understand the difference between the two structures we must look at the stacking of tetrahedra, ABAB... in wurtzite and ABCABC... in sphalerite (click here for illustration). The strong structural similarity suggests that the two structures should have similar stabilities. This is borne out by the observation that many compounds are known to exist in both structure types (ZnS, ZnSe, CdS, ZnO, CuI, CuCl, AgI, SiC, ...). The general wisdom is that sphalerite becomes increasingly favored as the covalent character of thee bonding increases. This may arise from the fact that strictly speaking the site symmetry is tetrahedral (43m) only in sphalerite.