review
advertisement

Review
Chapter 2 know general structures
Chapter 3
Much of this class deals with the partitioning
of an organic compound i between two
phases
A+B C
Keq = [C]/{[A][B]}
Keq = [ iphase1]/[ iphase 2]
When we deal with air liquid partitioning
KiaL = Cia/CiL
Octanol-water
Kiow = Cio/Ciw
Solid-water
Kid = Cis/Ciw
We will find that often for classes of
compounds
log Kid = a log Kiow + b
Why???
disp Gfor one mole
n2Di 1 n2D1 1
N A TSA / CA3I / 256 2
x 2
nDi 2 nD1 2
The concept of free energy comes from the
need to simultaneously deal with the
enthalpy energy and entropy of a system
at constant temp and pressure
G= H -TS
Equilibrium Constants
From free energies of reactants and
products
o
G RT ln
(PC )c (PD )d
(PA )a (PB )b
Equilibrium Constants and
Temperature
(G/T)P= -S
d(ln K eq )
dT
and (G/T)P= -S
Ho
RT 2
log K eq
Ho
(1 / T) const
2.303R
We than defined chemical potentials
N
i ( nGi )T , P , n
i 1
j
G i /n i = i
From the Gibbs Duhem equation we ultimately
showed
2i = 1i +RT ln f2i/f1i
fi pure liquid = i pure liquid piL*
Phase Transfer Processes
Consider a compound, i ,which is dissolved
in two liquids which are immiscible like water
and hexane.
RT ln fi hx /fiL*(pure liquid) = RT lnfi H2O /fiL*(pure liquid)
fi hx = fi H2O
We then went on to define an excess free
energy of solution
GiE1 RT ln i 1
and
GEi1 = RTln i1= HEi1-TSEi1
HEi1 is the particle molar excess enthalpy of
solution
and
SEi1 is the partial excess entropy
Chapter 4
We derived
ln pio
Hi 1
const
R T
then introduced concept of sub-cooled
liquid vapor pressures
At the boiling point is vapSTb constant?
H
const slope = S
T
Discussed estimation techniques for S and Tb to
be used in vapor pressures calculation
techniques
ln p *iL
SvapTb
R
*
ln piL
19(1
*
ln piL
ln PiS*
[ 1 .8 ( 1
Tb
T
) 0.8(ln b )]
T
T
Tb
) 8.5(ln Tb )]
T
T
( S fus ) (Tm Tamb )
R
Tamb
WE then looked at LC and GC techniques to
measure solid and liquid vapor pressures.
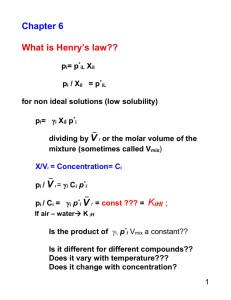
Chapter 6
_
pi / Ci = i p*i V l = KiHl
Wash out ratios or W
Usually defined as the conc. in rain/conc. In air
W = Ciw/Cia = 1/Kiaw
and calculated how fast rain could remove gas
phase organic contaminants from the
atmosphere based on their air water
partitioning or KiH
we then introduced the concept of saturated
Henry’s law values
sat
K iH
p sat
i
sat *
pl Vw
sat
w
C iw
and then asked how different iw
were ??
and iwsat
also KsatiH could be calculated from p*i and
Csatiw values from the appendix
we looked at the influence of salts,
where the Henry’s law for salt water is
K iH ,w , salt
K i s [ salt ]
sat
K iH 10
==============================
Example Problem: Consider a well sealed flask
with 100 ml of H2O and 900 ml air. At
equilibrium estimate the amount of
chlorobenzene in the air and in the water if the
sum (total) in both phases is 10 g.
fw = the fraction in the water phase
fw = chlorobenzene mass in water/total mass
fw
C w Vw
1
1
C w Vw Ca Va 1 Ca Va 1 K Va
H
Vw
C w Vw
Estimation techniques for Henry’s Law values
Experimental Measurement techniques
Used fugacities to model an multiphase
environmental system (Donald Mackay ES&T,
1979)
Chapter 5
Used the Hansen and UNIFAC techniques to
estimate activity coefficients
Xiwsat = 1/iwsat
(liquids)
Xiwsat = 1/iwsat
Csatiw =
*
pis
*
piL
*
p
is
Csatiw(L) *
piL
(solids)
*
p
Xiwsat = 1/iwsat ig*
piL
(gases)
Molar volumes could be estimated from
Mw/density and Csatiw (L), from regressions of
molar volume
As an example we estimated Csatiw (L), satiwand
GEiw for di-n-butyl phthalate
on page 1206, -log Csatiw= 4.36
Csatiw = Xi / Vmix= 1 /i Vmix
GEiw= RTlnI = 3483 J/ (molK)
We then discussed solubility in terms of the
cavity –iceberg theory
Effects of co-solvents on dissolved organics in
an organic/water solution
sat
fcN( h: w h:c )HSA
Xmix
log sat
2
.
303
RT
X
w
HSA = Hydrophobic surface area,
h:w=hydrophobic interfacial energy,
h:c=hydrophobic interfacial energy where the
solute contacts the organic,
fc = volume fract. of organic
Chapter 7
Kisw = Cis / Ciw solvent- water system
Kiow = Cio / Ciw octanol-water system
Water saturated octanol
Vmix =(0.79)(0.16) + (0.21)(0.018) = 0.12 L mol-1
We observed that
log Kihw = 1.21 log Kiow -0.43
ln Kiow= -a ln Csatiw+ b
log Kiow = a log t +b
Bioaccumulation and octanol water, Kiow
[ trout lipid] = 1.5x10-7 moles /g fish lipid
gas particle partitioning
in the air-particle system Kp = Kiow/Kiaw x const
Chapter 8
'
+
[
H
]
[
A
]
H+
*
log K ia log
[HA]
pKia = -log [Kia]
At ambient pH values (4-10) low pKia
compounds will be present in natural waters in
their ionized (dissociated form) as their anions.
log {[A-]/[HA]}
= pH – pK*ia
(Henderson-
Hasselbach equation)
at what point does pH = pKia?
described the amount present in the acid
(unionized or dissociated) form
a
1
pH-pK
ia
1+10
example, 2-nitrophenol has a pKia of 7.17. At a
pH of 7, what is the fraction in the
undissociated acid form?
Organic Bases
(BH+ is the conjugate acid of B)
Kia
H'+ [H+ ] B' [B]
BH [BH+ ]
from log {[A-]/[HA]} = pH – pKia
we calculated that the addition of small amts.
of a strong acid will not overly affect the pH of
the natural water.
The Hammett Correlation
log Ka= log KaH +I for benzoic acid systems
for other organic groups,
log (Ka / KaH) = i
looked at Nicotine partitioning