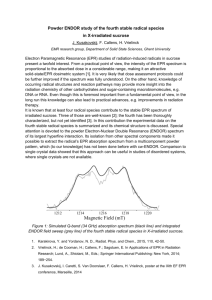
POLA_26500_sm_SuppInfo
advertisement

Supporting information for H-transfer reaction during decomposition of N-(2-methylpropyl)-N-(1-diethylphosphono-2, 2-dimethylpropyl)-N-oxyl radical (SG1)-based alkoxyamines. Mariya Edeleva,a Sylvain R.A. Marque,b* Kuanish Kabytaev, bYohann Guillaneuf,b Didier Gigmes,b and Elena Bagryanskayaa,c * a International Tomography Center SB RAS, Institutskaya 3A, Novosibirsk, 630090, Russia b Aix-Marseille Université, CNRS, UMR-7273, ICR, case 551, Avenue Escadrille Normandie– Niemen 13397, Marseille Cedex 20, France, sylvain.marque@univ-provence.fr c N.N.Vorozhtsov Novosibirsk Institute of Organic Chemistry SB RAS, Pr.Lavrentjeva 9, Novosibirsk, 630090, Russia, egbagryanskaya@nioch.nsc.ru Scheme 1. Mechanism of NMP. 2 NH N O P O OH O O N + O P O O P O + H 2O O O + HP(O)(OEt)2 N Scheme 2. Disproportionation reaction of Y-H. O N O P O + O + HP(O)(OEt)2 N O O H O Scheme 3. Intramolecular H-bonding in alkoxyamine 4. O O N O H P(O)(OEt)2 N OH + CO2 P(O)(OEt)2 Scheme 4. Possible decomposition of carbonic acid based-alkoxyamine. a) b) Figure 1. (a) 31P NMR of decomposition products of SG1 nitroxide in the presence of 20 eq. of PhSH. (b) Kinetics of Y-H decomposition (squares) and diethylphosphite (circles) formation during heating of SG1 nitroxide in the presence of 20 eq. of PhSH (benzene-d6, T = 75 °C). Ln [C]/[C]0 0 -3 -6 time, s 0 200 400 600 800 1000 Figure 2. (a) Semi-logarithmic plot of the kinetics of decomposition of alkoxyamines1, 2, and 4 (0.02 M solutions in benzene – d6) in the presence of scavenger at 75 oC. () Alkoxyamine 1; () alkoxyamine 2; () alkoxyamine 4; solid line – linear fit of the experimental data points. Figure 3SI. (a) The kinetics of alkene formation during decomposition of alkoxyamine 1 in the absence of scavenger at residual pressure 10-3 mbar in acidic (1.1 eq. of TFA) () and nonacidic conditions (●), (full line) – exponential fit. (b) 31P NMR before (lower) and after decomposition of alkoxyamine 1 in the absence of scavenger at residual pressure 10-3 mbar in acidic (middle) and non-acidic conditions (upper). EPR oximetry procedure using 9-deutero-triacetoxymetoxy-trityil radical Figure 4. Structure of the radical used for EPR oximetry. For EPR oximetry test a 0.25 mM solution of dAMT-3 radical was prepared according. The solution was degassed on oil pump according to the procedure used for alkoxyamine solutions (see experimental section). CW-EPR measurement of dAMT-3 radical signal were carried out on Bruker EMX; X-band spectrometer. g = 2.00351 Magnetic field / mT Figure 5. EPR spectrum of radical dAMT3 used for EPR oximetry in our experimental conditions (above) and at high vacuum (below). Hyperfine constant on CH2 group is equal to 42 mG. The additional line width obtained in in benzene was comparable with hfi constant. Line width is determined according to the following equation: lw = kD*[O2], where kD = 1010М–1s–1. 1 G in frequency units this is equals to 1,76*107rad/s. Than in frequency units lw=116 mG≈2 106rad/s. Let’s make the following assumptions: (i) kD = 1010М–1s–1, (ii) radical’s line width is fully determined by oxygen concentration. Than [O2]=lw/kD=2 10–4М. This is the upper limit of oxygen concentration in our experiment. Rate constant Table 1. Kinetic parameters used for simulation of alkoxyamine1decomposition kinetics. Case 1. Decomposition Case 2. Decomposition of 1 in the presence of 1 at P = 10-5 mbar of residual of oxygen (P = 10-3 mbar). value value kd 0.015 s-1a,b kc 1.5 106 M-1 s-1c kt 2 107 M-1 s-1d kcD 1.7 103 M-1 s-1a kNH 0.03 s-1 [O2] 0 mM 0.1 mMe k1 - 1 .107 M-1s-1f k2 - 104M-1s-1g k3 - 5 .103s-1g k4 - 1. 105s-1h k1* - 1.5.103 s-1 a i This work. bRef. 26c See text and ref. 46. d Ref. 47. e See text and SI. f See text. gRef. 52. hRef. 48. i It was accounting for k1 – k4 values. See text.