BIO 2724 LAB 2 - OSMOSIS AND TONICITY
advertisement

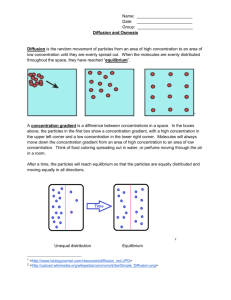
Lab 2.1 LAB 2: Passive transport in erythrocyte membranes Objectives - after completing this lab you should be able to: 1) explain the physical basis for and the result of diffusion 2) explain osmosis and osmotic pressure 3) explain the difference between diffusible (penetrating) vs nondiffusible (nonpenetrating) solutes and their effect on the rate of osmosis 4) define tonicity and explain the effect of solutions of different tonicities on RBCs 5) describe the effect of molecular size and lipid solubility on rate of solute diffusion through a membrane 6) explain the relationship between the rate of osmosis and the time it takes for hemolysis to occur Safety: 1) Wear gloves and safety glasses or goggles when handling blood suspensions and chemicals. A. Passive Transport 1) How is the relationship between solutes and solvents described? The simplest method is the percent solution--this refers to the number of grams of solute per 100 mL of solution. For example, a 5% glucose solution contains 5 g glucose per 100 mL solution. A 0.9% NaCl solution contains 0.9 g NaCl per 100 mL solution. We also use molar solutions in lab. A one molar solution of any substance contains 1 mole of solute in 1 liter of water. One mole of a solute equals the molecular weight of the solute in grams. A one molar (1M) solution of glucose would contain 180 g glucose/liter of water. A one molar (1M) solution of NaCl would contain 58.5 g NaCl/liter of water. In terms of molarity, 1M glucose is equivalent to 1M NaCl. Solute concentrations in the body fluids are relatively low, so their concentrations are measured in milligrams per 100 mL (mg/dL or mg%) or in millimoles (mM). One milligram is what fraction of one gram? _________________. One millimole is what fraction of one mole? __________________ If a 1 M solution contains 1 mole solute in 1 liter of water, a 1 mM solution would contain what fraction of a mole of solute in 1 liter of water? _______ 2) What is the relationship between osmosis, osmolarity and tonicity? Osmosis is the diffusion of water through a selectively or semipermeable membrane, and is caused by an unequal distribution of solutes on the two sides of the membrane. Water is “drawn” Lab 2.2 towards the side of the membrane with the higher solute concentration. When the solute concentrations on the two sides of the membrane are equal, no osmosis occurs. Osmolarity is a measure of concentration based on the number of particles per liter of solution. Molecules like glucose do not dissociate in water, and only contribute one particle per molecule. NaCl does dissociate in water (into Na+ and Cl-), so there are two particles per molecule in solution. if osmolarity (OsM) = # particles/mole X # moles/liter, then OsM of 1 M glucose = 1 particle/mole X 1 mole/liter = 1 OsM, and OsM of 1 M NaCl = 2 particles/mole X 1 mole/liter = 2 OsM So, two solutions can have the same molarity but different osmolarities. The higher the osmolarity, the greater the osmotic pressure of the solution. The greater the osmotic pressure, the more water is pulled across the membrane. Because solute concentrations in the body are low they are measured in milli-osmoles (mOsm) instead of Osm. The osmolarity of body fluids is around 300 mOsm. Tonicity describes the effect of an extracellular solution on the volume of a cell and is determined by the relative concentration of nonpenetrating solute molecules. An isotonic solution has the same concentration of nonpenetrating solutes as the ICF (iso- means same). Some percent solutions that are isotonic to ICF are 0.9% NaCl and 5.0% glucose. A hypotonic solution has a lower concentration of nonpenetrating solutes than the ICF (hypo- means below or under), and a hypertonic solution has a higher concentration than the ICF (hyper- means above or over). a. If you know the tonicity of a solution you can predict its effect on cells. If you put RBCs into an isotonic solution, the nonpenetrating solute concentrations on both sides of the membrane are the same. (For the purpose of these examples, we will assume that the osmolarity of the solutions is based on nonpenetrating solute concentrations.) ECF = 300 mOsm Will osmosis occur? _______________________ Will the cell volume change?_________________ ICF = 300 mOsm Lab 2.3 If you put RBCs into a hypotonic solution, the nonpenetrating solute concentration on the outside of the cell is lower than on the inside. ECF = 250 mOsm Will osmosis occur? __________________ In which direction? ___________________ ICF = 300 mOsm Will the cell volume increase or decrease? When RBCs swell up their membrane becomes very leaky, and the hemoglobin leaks out, leaving an empty plasma membrane (ghost). This phenomenon is called hemolysis (from hemo - blood and lysis - disintegration) even though the cell does not actually rupture. You just cannot see the cells any more because there is nothing left inside them. The hemoglobin is evenly distributed in the solution instead of being inside the RBC membranes. If you put RBCs into a hypertonic solution, the nonpenetrating solute concentration on the outside of the cell is higher than on the inside ECF = 350 mOsm Will osmosis occur? ___________________ In which direction? ____________________ ICF = 300 mOsm Will the cell volume increase or decrease? When RBCs shrink their membrane shrivels up and they look lumpy. This is called crenation. b. You can also use RBCs to determine the relative tonicity of a solution. If you put RBCs into an isotonic solution they will neither crenate nor hemolyze. If you put them into a hypotonic solution they will ___________, and if you put them into a hypertonic solution they will ____________. Lab 2.4 We can tell just by looking at suspensions of RBCs whether the cells are intact (normal or crenated) or hemolyzed. (You can’t tell if RBCs are normal or crenated without looking at them under a microscope.) A suspension of intact RBCs is cloudy, and you cannot see through it easily. A suspension of hemolyzed RBCs is red but transparent, and you can see through it. A suspension of hemolyzed RBCs is red but transparent, and you can see through it. intact cells hemolyzed cells 3) RBCs can be used a) to measure the rate of osmosis, b) to detect differences in the osmolarity of solutions, and c) to look for permeability changes in the RBC membrane. When RBCs are incubated in solutions, penetrating solutes can enter the cell. As they do so, they draw water with them by osmosis. As water enters the cell, the cell swells and hemoglobin begins to leak out (hemolysis). The faster the solutes enter, the more quickly hemolysis occurs. What effect would nonpenetrating solutes have on osmosis and hemolysis? Absorbance is higher in RBC suspensions that contain whole cells (isotonic and hypertonic solutions) and lower in RBC suspensions that contain hemolyzed cells (hypotonic solutions). Another way to say this is that transmittance is lower in RBC suspensions of whole cells and higher in RBC suspensions of hemolyzed cells. a. There is a direct relationship between the length of time required for hemolysis to occur and the rate of osmosis into the cell. The higher the rate of osmosis, the faster hemolysis occurs. Therefore, the time it takes for hemolysis to occur can be used to indirectly measure the rate of osmosis. You can do this by monitoring one tube over a period of time while it is in the spectrophotometer. Absorbance should be high while the cells are still intact, but it should decrease when the cells hemolyze. The sooner the absorbance decreases, the higher the rate of osmosis. Lab 2.5 b. The osmolarity of various solutions can be assessed by comparing absorbance after adding RBCs to the solutions. You measure absorbance in each tube--the lower the absorbance, the greater the degree of hemolysis caused by the solution. c. The amount of osmosis, measured indirectly by monitoring hemolysis, can be used to detect changes in RBC membrane permeability under various conditions such as changes in temperature, pH, and membrane stability. You would use a control tube containing RBCs in isotonic saline and experimental tubes in which conditions have been altered. Experimental variables that increase RBC permeability would have higher rates of osmosis and therefore greater hemolysis. 4) Why use red blood cells as models to study osmosis? a. RBCs are also easy to obtain and work with and the molecular structure of the RBC membrane is well understood. b. RBCs are capable of either crenation or hemolysis, so they will respond to solutions of different osmolarities or tonicities. By examining the effect of a solution on the cells, we can tell whether it is isotonic, hypotonic, or hypertonic. c. We could use artificial membranes to detect tonicity, but they are made of cellulose with known pore sizes. They do not contain channels, carriers, or pumps, so they cannot be used to study membrane transport using those methods. 5) How do lipid solubility and molecular size affect the rate of diffusion of solute molecules into RBCs? Lipid soluble (nonpolar) molecules enter cells by diffusing through the lipid bilayer, but molecules that are not lipid soluble (polar) have to pass through channels. The rate of diffusion of nonpolar molecules depends on how soluble they are in lipids. The rate of passage of polar molecules depends on the size of the molecule (which is proportional to its molecular weight). In general, small nonpolar molecules would pass though a membrane faster than larger, polar molecules. Lab Activity Instructions: Effect of molecular size and lipid solubility on rate of osmosis. You will be given 4 solutions labeled A-D. You will observe their effects on hemolysis (and indirectly on osmosis) to identify the solute present in each solution. You will do this by comparing the rate of entry of the solute for each solution and using the information in the table below to infer the identity of the solutes based on their predicted effects. solute concentration molecular penetration polarity weight glucose 0.5 M 180 permeating polar glycerol 0.5 M 92 permeating nonpolar sucrose 0.5 M 342 nonpermeating polar Lab 2.6 urea 0.5 M 60 permeating nonpolar 1) Label 6 test tubes: A, B, C, D, H2O and saline (0.9%). 2) Measure 3 mL of the appropriate solution into each tube. Use one of your pipets for measuring water first, then use it for one of the solutions. NOTE: Always swirl the RBC suspension immediately before dispensing. 3) Add 1 drop of blood to the saline tube and mix by inverting gently. This solution should remain cloudy red. If it becomes clear red, notify the instructor. 4) Add 1 drop of blood to the H2O tube and mix by inverting gently. This solution should be clear red (not cloudy). Have the instructor examine both tubes before proceeding. 5) Add 1 drop of blood to tube A and immediately mix by inverting gently. Record the exact time when the blood was added. Watch the tube, checking every 10 seconds for the first 2 minutes, then every 60 seconds through minute 15 to see if it becomes clear. Record status in the data chart at each time check. 6) Repeat step 5 for tubes B, C and D, but only if you have enough people in your group for each person to time one tube. 7) Cleanup and disposal: a. tubes 1. empty tube into hazardous waste container 2. rinse tube with 1 mL water and empty into hazardous waste container 3. put empty tube into broken glass box b. gloves and plastic pipets go into regular trash c. glass pipets go into tub in sink d. wipe table with soapy water Lab 2.7 time blood added unknown A unknown B unknown C unknown D 10 sec 20 sec 30 sec 40 sec 50 sec 1 min 10 sec 20 sec 30 sec 40 sec 50 sec 2 min 3 min 4 min 5 min 6 min 7 min 8 min 9 min 10 min 11 min 12 min 13 min 14 min 15 min Questions 1. What kind of solute enters the RBC faster: small or large? 2. What kind of solute enters the RBC faster: polar or nonpolar? 3. What kind of solute should cause hemolysis faster: small or large? 4. What kind of solute should cause hemolysis faster: polar or nonpolar? Lab 2.8 5. Which unknown solute was the smallest and least polar: A 6. Which unknown solute was nonpermeating: A B C B C D D 7. Which solution should cause hemolysis sooner (Assume the membrane is equally permeable to all ions); 0.5 M NaCl or 0.5 M CaCl2? 8. Osmolarity measures the number of solute _____________ per liter of solution. 9. Tonicity is determined by the relative concentration of ________________ solute molecules in an extracellular solution. 1) Observations: a. Permeating solutes enter RBCs (faster/slower) than nonpenetrating solutes. b. Small solutes enter RBCs (faster/slower) than large solutes. c. Polar solutes enter RBCs (faster/slower) than nonpolar solutes. d. The higher the rate of osmosis, the (higher/lower) the rate of hemolysis. 2) Predictions: a. If osmosis occurs in solutions of small solutes before it occurs in solutions of large solutes, then hemolysis should occur in glucose (before/after?) it occurs in glycerol. b. If osmosis occurs in solutions of nonpolar solutes before it occurs in solutions of polar solutes, then hemolysis should occur in glycerol (before/after?) it occurs in glucose. c. If osmosis does not occur in solutions of nonpermeating solutes, then hemolysis (will/will not) occur in the sucrose solution. 3) Based on the information you have been given, rank the 4 solutes on their expected rate of entry into the RBCs: fastest: slowest: .