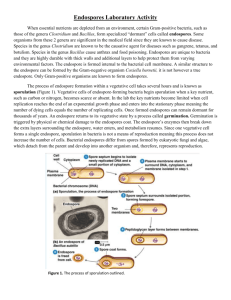
The Endospore stain
advertisement

DIFFERENTIAL STAINING: THE ENDOSPORE STAIN Vegetative cells are bacteria that are actively growing, metabolizing and dividing. When vegetative cells are subjected to environmental stresses such as nutrient deprivation they eventually die. However, some bacteria such as the Bacillus spp. and the Clostridium spp can circumvent the problems associated with environmental stress by forming endospores. Endospores are dormant or metabolically inactive forms of a bacterium that allow it to survive the harsh environmental conditions. Spores are resistant to heat, UV radiation and chemicals because they are comprised of a tough proteinaceous covering called keratin. A mature endospore contains a complete set of the genetic material (DNA) from the vegetative cell, ribosomes and specialized enzymes. Endospores may be located in the middle of the bacterium (central), at the end of the bacterium (terminal) and near the end of the bacteria (subterminal) and may be spherical or elliptical. Mature endospores are released from the vegetative cell to become free endospores. When the free endospores are placed in an environment that supports growth, the endospores will revert back to a vegetative cell in a process called germination. It should be noted that unlike the process of binary fission observed with vegetative cells, endospore formation is not a reproductive process but a process of differentiation that provides the bacteria with a mechanism for survival. Most endospore forming bacteria are found in soil or aquatic environments. However, some species of Bacillus and Clostridium have medical significance. Clostridium perfringens, C. botulinum and C. tetani are the causative agents of gas gangrene, botulism and tetanus, respectively. Bacillus anthracis and Bacillus cereus are the causative agents of anthrax and a self limiting food poisoning, respectively. In this exercise, you will perform an endospore stain on Bacillus spp. a nonpathogenic, spore forming Gram positive rod. The instructor will provide a sample containing bacteria that have been grown under different conditions: bacteria grown for 4-8 hours, vegetative bacteria; bacteria grown for 24-48 hours, vegetative bacteria containing endospores and bacteria grown for 72 hours; mature free endospores. A differential staining technique (the Schaeffer-Fulton method) is used to distinguish between the vegetative cells and the endospores. A primary stain (malachite green) is used to stain the endospores. Because endospores have a keratin covering and resist staining, the malachite green will be forced into the endospores by heating. In this technique heating acts as a mordant. Water is used to decolorize the cells; as the endospores are resistant to staining, the endospores are equally resistant to de-staining and will retain the primary dye while the vegetative cells will lose the stain. The addition of a counterstain or secondary stain (safranin) is used to stain the decolorized vegetative cells. When visualized under microscopy the cells should have three characteristics: the vegetative cells should appear pink, the vegetative cells that contain endospores should stain pink while the spores should be seen as green ellipses within the cells. Mature, free endospores should not be associated with the vegetative bacteria and should be seen as green ellipses. 1 The endospore stain: 1. Prepare a heat fixed smear on a clean microscope slide of the bacteria as shown by the instructor. To this end the instructor will provide 3 plates with endospore producing bacteria that have been grown on the plates for 4-8 hours, 24-48 hours and 72 hours. Place a small drop of sterile water on the slide and add a very small aliquot of the bacteria to the water drop with an inoculating needle. Use a sterile inoculating loop to make a very thin smear of the bacteria on the surface of the slide. Allow the smear to dry completely. 2. Place a small piece of blotting paper over the smear and place the slide (smear side up) on a staining rack that has been placed over a boiling water bath in the corner of the room. 3. Put on gloves and leave such gloves on until you finish staining the specimen. 4. Completely cover the blotting paper that covers the smear with malachite green and allow the slide to heat thoroughly over the water bath for 10 minutes. Be certain to replace any dye that has evaporated with fresh dye. 5. After 10 minutes carefully remove the slide from the rack using a clothespin, tip off any extra stain into a pan that is adjacent to the bath and take the slide back to your bench. 6. Remove the blotting paper and allow the slide to cool to room temperature for 2 minutes. 7. Use water to wash the malachite green from both sides of the microscope slide. 8. Stain the smear with safranin for 2 minutes. 9. Rinse both side of the slide to remove the secondary stain and use the booklet of bibulous paper to blot the slide. 10. Save your slide for the observation next lab period. Observe the bacteria under 1000X (oil immersion) total magnification. At this point you should be able to identify the vegetative cells, the endospore containing cells and the free endospores. Draw a picture of what you observe with microscopy noting the color of the cells and the spores. 2 Do the spores appear elliptical or spherical? Are the spores central, terminal or subterminal? How many spores are present in each bacillus? 3