Oil Spill Activity
advertisement

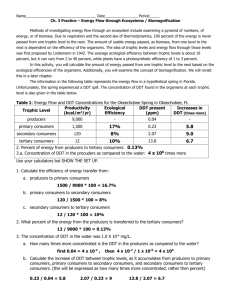
Oil Spill Activity Applications and Extensions Answers 1) What two physical properties of oil and water cause oil spills to have such disastrous effects on the environment? ~ Density and Solubility - Oil is less dense than water so it floats along the surface of the water. This prevents sunlight from penetrating the water, which limits photosynthesis of marine plants and phytoplankton. Surface animals (birds, otters, turtles, seals, etc.) run the risk of ingesting oil, which causes dehydration and impaired digestion, or becoming coated in oil, which affects their insulation abilities - Oil is also insoluble in water, so it slowly spreads along the surface until it is removed. 2) Because a body of water is a closed system, why is it important to keep oil out? - The closed body of water cannot get rid of the oil on it’s own. The oil will spread along the surface of the water and eventually wash up along the shoreline 3) Why is clean up so difficult? - there is no one effective way to deal with oil spills - clean up methods are slow and very costly 4) Why can clean up also cause damage? - toxic chemicals may be used to clean up the oil that pollute the environment and harm animals - controlled burns add air pollution - animals may be caught or trapped in clean up contraptions 5) What can be done to prevent oil from leaking into water bodies? - - - limit usage/demand on oil so that there is less being transferred (use more “green” methods of transportation to reduce need for cars) more regulations placed on how much oil can be transferred at a time and the ways in which it can be transferred more safety checks and inspections for transportation vehicles, pipes, storage facilities, etc. more funding to find more effective cleaning processes prevention of spills as opposed to reacting to disasters 6) Read through Page 26 and 154 in your textbook a. What property of DDT allows it to continue to accumulate I the fatty tissue of mammals despite its ban by the Canadian government in the 1980s? - solubility – DDT is soluble in fat, but not in water so animals can’t excrete it very well and it continues to accumulate in fatty tissue - bioaccumulation – as time wears on, small amounts of DDT remain in animals and build up - biomagnification – contaminated animals that are eaten contaminate their predators – the higher up the food chain you go, the more concentrate the DDT beomes 7) Read through page 133 and 145-146 in your textbook: a. What properties of polyethylene make it a useful compound in a variety of products? - plastic that is used in many products due to it’s flexibility, durability and versatility (water bottles, bullet proof vests, etc.) b. What properties of polyethylene pose problems for the environment? - durable – doesn’t break down, but accumulates and pollutes the environment - toxic processes used to manufacture products