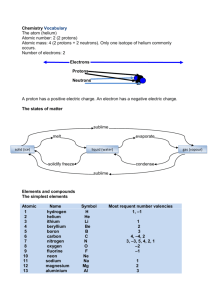
Created by the HTML-to-RTF Pro DLL .Net 4.6.10.19 Q1. Soap can
advertisement

Q1. Soap can be made by reacting fats with sodium hydroxide solution. fat + sodium hydroxide → soap + glycerol The diagram shows a laboratory experiment to make soap. From the information in the diagram, give two factors which increase the rate of this reaction. In each case explain, in terms of particles, why the rate of reaction increases. Factor 1 ................................................................................................................................ ............................................................................................................................................... Reason .................................................................................................................................. ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... Factor 2 ................................................................................................................................. ............................................................................................................................................... Reason .................................................................................................................................. ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... (Total 7 marks) Page 1 Q2. Iron is used (as steel) to make the body panels for cars. The iron panels have to be bendable so that they can be pressed into the shape required, but must also be strong. The panels must also be able to conduct electricity because they form part of the electrical circuits of the car. (a) Iron is a typical metal. Describe the structure and bonding in a metal such as iron. You may use a diagram if you wish. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (4) (b) Explain how the structure and bonding of iron: (i) allows the body panels to conduct electricity; ........................................................................................................................... (2) Page 2 (ii) allows the body panels to be bent into shape; ........................................................................................................................... (1) (iii) gives the body panels strength. ........................................................................................................................... (1) (Total 8 marks) Q3. Some drain cleaners contain a mixture of sodium hydroxide and powdered aluminium. When the mixture is poured into a drain it mixes with water and a chemical reaction takes place. The heat from the reaction helps to melt grease in the drain. Hydrogen gas is produced which stirs up the particles and helps to unclog the drain. (a) Balance the equation for the reaction. 2Al(s) + ........ NaOH(aq) + ......... H2O → .........NaAl(OH)4(aq) + 3H2 (2) (b) Why do the solid sodium hydroxide and aluminium powder not react when stored in a sealed container? ..................................................................................................................................... (1) (c) Sodium hydroxide is a strong alkali and would react with any acids in the drain. (i) Name the ion produced when any alkali is dissolved in water. ........................................................................................................................... (1) (ii) Name the ion produced when any acid is dissolved in water. ........................................................................................................................... (1) Page 3 (iii) Name the compound formed when these ions react with each other. ........................................................................................................................... (1) (Total 6 marks) Q4. Sodium hydroxide, hydrogen and chlorine can all be made in one industrial process. Electricity is passed through aqueous sodium chloride solution (brine). The diagram below shows a cell that can be used for this process. (a) Name A, B and C. Gas A ......................................................................................................................... Gas B ......................................................................................................................... Solution C .................................................................................................................. (2) (b) Balance the equations for the reactions at the electrodes. (i) .......... Cl– – ............ e– → Cl2 (ii) .......... H+ + ............ e– → H2 (2) (c) Name the compound in this cell which produces the hydrogen ions. ..................................................................................................................................... Page 4 (1) (d) Which type of particles must be able to pass through the barrier to allow the electrolysis to take place? ..................................................................................................................................... (1) (Total 6 marks) Q5. Sulphur hexafluoride is a colourless, odourless, non-flammable gas, which is insoluble in water and extremely unreactive. It is used as an insulator in high voltage transformers and switchgear. The diagram below represents a molecule of sulphur hexafluoride. (a) What type of chemical bond holds the sulphur and fluorine atoms together in sulphur hexafluoride molecules? ..................................................................................................................................... (1) (b) Explain why sulphur hexafluoride has a low boiling point. ..................................................................................................................................... ..................................................................................................................................... (2) (c) Explain how three of the properties of sulphur hexafluoride make it suitable for use as an insulator inside electrical transformers. Page 5 Property 1: .................................................................................................................. Explanation: ............................................................................................................... ..................................................................................................................................... Property 2: .................................................................................................................. Explanation: ............................................................................................................... ..................................................................................................................................... Property 3: .................................................................................................................. Explanation: ............................................................................................................... ..................................................................................................................................... (3) (Total 6 marks) Q6. Calcium and magnesium are elements. They are found in the Earth’s crust as compounds, often carbonates and sulphates. Magnesium is also found as its chloride. (a) Calcium and magnesium are in the same Group in the Periodic Table. State which Group this is. ..................................................................................................................................... (1) (b) Use the Data Sheet to help you to answer this question. (i) Write the chemical formula of magnesium chloride. ........................................................................................................................... (1) (ii) Name the type of bonding in magnesium chloride. ........................................................................................................................... (1) (Total 3 marks) Page 6 ## Sando-K is a medicine. It is given to people whose bodies contain too little of a particular element. Sando-K is a mixture of two compounds. The formulae of the two compounds are given below. KHCO3 (a) KC1 Which metal do people given Sando-K need? ..................................................................................................................................... (1) (b) Sando-K contains the ion, CO32–. Which gas would be produced if a dilute acid was added to Sando-K? (The Data Sheet may help you to answer this question.) ..................................................................................................................................... (1) (c) The compounds in Sando-K contain ions. Complete the two sentences below. Atoms change into positive ions by ....................................... one or more ............................................................. . Atoms change into negative ions by ......................................... one or more .................................................... . (4) (d) Electricity can be used to show that an aqueous solution of Sando-K contains ions. (i) Draw a diagram of an apparatus that you could use to prove that Sando-K contains ions. Page 7 (4) (ii) Explain, as fully as you can, what would happen when the electricity is switched on. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (3) (Total 13 marks) Q8. Diesel oil is obtained from crude oil. It can be used as a fuel for car engines. The diagram below represents a compound found in diesel oil. (a) What is the formula of this compound? ..................................................................................................................................... (1) (b) Each of the lines on the diagram above represents a covalent bond. What is a covalent bond? ..................................................................................................................................... ..................................................................................................................................... (2) (Total 3 marks) Page 8 Q9. The following passage was taken from a chemistry textbook. Germanium is a white, shiny, brittle element. It is used in the electronics industry because it is able to conduct a small amount of electricity. It is made from germanium oxide obtained from flue dusts of zinc and lead smelters. The impure germanium oxide from the flue dusts is changed into germanium by the process outlined below. STEP 1 The germanium oxide is reacted with hydrochloric acid to make germanium tetrachloride. This is a volatile liquid in which the germanium and chlorine atoms are joined by covalent bonds. STEP 2 The germanium tetrachloride is distilled off from the mixture. STEP 3 The germanium tetrachloride is added to an excess of water to produce germanium oxide and hydrochloric acid. STEPS 1 to 3 are repeated several times. STEP 4 The pure germanium oxide is reduced by hydrogen to form germanium. (a) Balance the equation below which represents the reaction in step 1. GeO2 + ............ HCl → GeCl4 + ............ H2O (1) (b) Write a word equation for the reaction in step 3. ..................................................................................................................................... (1) (c) Suggest why steps 1 to 3 are repeated several times. ..................................................................................................................................... ..................................................................................................................................... (1) (d) The equation which represents the reaction in step 4 is shown below. Page 9 GeO2 (i) + 2H2 → Ge + 2H2O Explain what is meant by the term ‘reduced’. ........................................................................................................................... ........................................................................................................................... (1) (ii) Calculate the mass of germanium which could be made from 525 g of germanium oxide. (Relative atomic masses: Ge = 73; O = 16). ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... Mass ......................................... g (3) (e) Germanium is difficult to classify as either a metal or a non-metal. (i) Give as much evidence as you can from the information in this question to support the view that germanium is a metal. Explain your answer as fully as you can. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (3) (ii) Give as much evidence as you can from the information in this question to support the view that germanium is a non-metal. Explain your answer as fully as you can. ........................................................................................................................... Page 10 ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (3) (Total 13 marks) Q10. (a) The electronic structure of a sodium atom can be written 2,8,1. Write the electronic structure of a potassium atom in the same way. ..................................................................................................................................... (1) (b) The electronic structure of a sodium atom can also be represented as in the diagram below. (i) Draw a similar diagram for a fluorine atom. (ii) Draw similar diagrams to show the electronic structure of the particles in sodium fluoride. Page 11 (4) (Total 5 marks) Q11. Sodium carbonate is a useful chemical that can be made from sodium chloride. (a) The flow chart below shows one way in which sodium carbonate can be made. (i) Write the formula of sodium carbonate. Use the Data Sheet to help you to answer this question. ........................................................................................................................... (1) Page 12 (ii) 1. Give one example of a thermal decomposition reaction shown in the flow chart. ................................................................................................................ ................................................................................................................ (1) 2. Explain what is meant by a thermal decomposition reaction. ................................................................................................................ ................................................................................................................ (2) (iii) Name one substance that is recycled in this process. ........................................................................................................................... (1) (b) When sodium carbonate solution is added to zinc sulphate solution a white solid is precipitated. (i) Use the Data Sheet to help you to name the white solid that is produced in this reaction. ........................................................................................................................... (1) (ii) State why this solid is formed. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (1) (Total 7 marks) Q12. (a) In an industrial process electricity is passed through a solution of sodium chloride in water. A student set up the apparatus shown below to investigate this Page 13 process. (i) Name gas X. ........................................................................................................................... (1) (ii) Complete the half equation for the production of chlorine gas during the electrolysis. ............ Cl– → ............ e– → Cl2 (1) (iii) The student found that the solution left in the cell was alkaline. Which ion makes the solution alkaline? ........................................................................................................................... (1) (iv) Name the useful substance that can be obtained from the solution in the cell. ........................................................................................................................... (1) (b) Sodium carbonate is another useful chemical that can be made from sodium chloride. The flow chart below shows one way in which sodium carbonate can be made. Page 14 (i) Write the formula of sodium carbonate. Use the Data Sheet to help you to answer this question. ........................................................................................................................... (1) (ii) Salt is one raw material used in this process. Name one other raw material used in this process. ........................................................................................................................... (1) (iii) Sodium carbonate is one of the products of this process. Name one other product. ........................................................................................................................... (1) (iv) 1. Give one example of a thermal decomposition reaction shown in Page 15 the flow chart. ................................................................................................................ ................................................................................................................ (1) 2. Explain what is meant by a thermal decomposition reaction. ................................................................................................................ ................................................................................................................ (2) (v) Name one substance that is recycled in this process. ........................................................................................................................... (1) (c) When sodium carbonate solution is added to zinc sulphate solution a white solid is precipitated. (i) Use the Data Sheet to help you to name the white solid that is produced in this reaction. ........................................................................................................................... (1) (ii) State why this solid is formed. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (1) (Total 13 marks) Q13. Calcium oxide (quicklime) is made by heating calcium carbonate (limestone). calcium carbonate → calcium oxide + 100g ? Page 16 carbon dioxide 44g (a) 44 grams of carbon dioxide is produced when 100 grams of calcium carbonate is heated. Calculate the mass of calcium oxide produced when 100 grams of calcium carbonate is heated. .................................................................................................................................... mass ......................... g (1) (b) What mass of carbon dioxide could be made from 100 tonnes of calcium carbonate? mass ....................... tonnes (1) (Total 2 marks) Q14. The extract below was taken from a leaflet on the uses of platinum. One of the uses described was in making electrodes for spark plugs in car engines. The spark plug produces the spark which ignites the fuel in the engine. Spark Plugs The electrodes in a spark plug have to conduct electricity very well. Since they project into the combustion chamber of the engine, they must also be able to withstand extremely high temperatures in a very corrosive atmosphere. Nickel-based plugs have been produced for many years. They only last a fairly short time. As the electrodes wear, combustion becomes less efficient and the petrol is not burnt completely. Platinum and other precious metals can now be used in spark plugs. These last much longer and are more efficient. This can help to reduce air pollution. The table below gives some information about platinum and nickel. MELTING POINT (° C) BOILING POINT (° C) Page 17 POSITION IN REACTIVITY SERIES COST (£/kg) nickel 1455 2920 Higher than gold 2.5 platinum 1769 4107 below gold 6110 (a) Compare nickel and platinum for use in making the electrodes in spark plugs. A good answer should give advantages and disadvantages of each metal linking these to the properties of the metals. Marks will be given for the way in which you organise your answer. You will need a sheet of lined paper. (8) (b) (i) Describe the structure and bonding in metals. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (3) (ii) Explain why metals such as nickel and platinum are good conductors of electricity. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (2) (Total 13 marks) Page 18 Q15. (a) This label has been taken from a packet of Andrews Antacid. (i) Write the simplest ionic equation which represents a neutralisation reaction. ........................................................................................................................... (1) (ii) Chewing the tablet cures indigestion faster than swallowing the tablet whole. Explain why. ........................................................................................................................... ........................................................................................................................... (1) (iii) Write the formula of the magnesium compound present in Andrews Antacid. You may find the Data Sheet helpful. ........................................................................................................................... (1) (b) The active ingredients in the Antacid react with hydrochloric acid in the stomach to give salts, water and carbon dioxide. A student investigated how quickly the tablets react with excess hydrochloric acid. Page 19 40 cm³ of dilute hydrochloric acid were placed in a conical flask. The flask was placed on a direct reading balance. Two Antacid tablets were quickly added to the flask. The apparatus was weighed immediately. At the same time, a stop clock was started. The mass was recorded every half minute for 5 minutes. The results are shown in the table below. The main active ingredient in Andrews Antacid is calcium carbonate. (i) Balance the equation which represents the reaction between calcium carbonate and hydrochloric acid. CaCO3(s) + .......... HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g) (1) (ii) State the meaning of the symbol “(aq)”. ........................................................................................................................... (1) (iii) Why does the mass of the flask and contents decrease? ........................................................................................................................... (1) (c) (i) Plot the results on the graph below and draw a smooth curve to show how the mass of the flask and its contents changes with time. Label this curve “A”. Page 20 (3) (ii) One of the results does not appear to fit the pattern. Circle this result on the graph. (1) (d) The student did a second experiment. The only change was that the acid was twice as concentrated. On the graph, sketch a second curve to show a possible result for this experiment. Label this curve “B”. (2) (Total 12 marks) Page 21 Q16. Ammonium nitrate is an important fertiliser. It is made by reacting nitric acid with the alkali ammonia. (i) State the type of reaction taking place. ........................................................................................................................... (1) (ii) The equation for this reaction is: NH3 + HNO3 → NH4NO3 Calculate the number of tonnes of ammonium nitrate that can be made from 68 tonnes of ammonia. (Relative atomic masses: H = 1, N = 14, O = 16) ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (3) (Total 4 marks) Q17. 280 000 tonnes of magnesium are produced in the world each year. The pie chart below shows the ways in which magnesium is used. Page 22 (a) (i) Use the pie chart to calculate the percentage of magnesium used to make aluminium alloys. ....................................... % (1) (ii) How many tonnes of magnesium are used to make aluminium alloys each year? ....................................... tonnes (1) (b) Magnesium is produced by the electrolysis of molten magnesium chloride. The reactions which take place at the electrodes are represented by the equations below. Mg2+ + 2e– → Mg 2Cl– – 2e– → Cl2 (i) Calculate the mass of chlorine produced when one kilogram of magnesium is made. (Relative atomic masses: Mg = 24, Cl = 35.5) Page 23 ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (3) (ii) Give a use for chlorine. ........................................................................................................................... (1) (Total 6 marks) Q18. The hydrogen halides (hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide) are important chemicals. The diagram below represents a molecule of hydrogen chloride. (i) What type of particles are represented by the crosses (X)? ..................................................................................................................................... (1) (ii) What type of chemical bond holds the atoms in this molecule together? ..................................................................................................................................... (1) Page 24 (iii) Would you expect hydrogen chloride to be a gas, a liquid or a solid, at room temperature and pressure? Explain your answer. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (3) (Total 5 marks) Q19. Limestone (CaCO3) is a raw material. On strong heating it is converted to calcium oxide which is a very useful substance. (a) Calculate the formula mass (Mr) of calcium carbonate. ..................................................................................................................................... Mr of calcium carbonate = ............................................... (2) (b) About 60 million tonnes of calcium oxide is made in Britain each year. Calculate the mass of calcium carbonate needed to make this amount of calcium oxide. .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... Mass of calcium carbonate needed = .............................. million tonnes (4) Page 25 (c) Water is added to some of the calcium oxide produced in a process known as ‘slaking’. The product of this reaction is used to make plaster. CaO(s) + H2O(1)→ Ca(OH)2(s) (i) Give the chemical name of Ca(OH)2. .......................................................................................................................... (1) (ii) What is the physical state of the Ca(OH)2 formed in the reaction? .......................................................................................................................... (1) (Total 8 marks) Q20. Silicon is an extremely important element. More than a million tonnes of silicon are produced each year. Silicon is made by reducing silicon oxide (sand) with carbon (coke). (a) (i) Complete the diagram below to show the arrangement of electrons in an atom of silicon. The Data Sheet may help you with this question. (2) (ii) Which electrons in the silicon atom take part in chemical reactions with other atoms? .......................................................................................................................... .......................................................................................................................... (1) Page 26 (iii) What features of all the atoms of the elements in group 4 of the Periodic Table might give them similar chemical properties? .......................................................................................................................... .......................................................................................................................... (1) (b) Silicon is difficult to classify as a metal or a non-metal because it has properties which resemble both. Some of the properties of silicon are listed below. • • • • • • • • Silicon is a shiny blue/grey solid. Silicon is placed in Group 4 of the Periodic Table. Silicon has a relative atomic mass of 28. Silicon has a very high melting point (1410ºC). Silicon has a very high boiling point (2355ºC). Silicon conducts electricity. Silicon oxide will neutralise alkalis. Silicon forms compounds in which the silicon atoms are bonded to other atoms by covalent bonds. (i) Select two properties from the list above in which silicon resembles a metal. 1. ..................................................................................................................... 2. ..................................................................................................................... (2) (ii) Select two properties from the list above in which silicon resembles a non-metal. 1. ..................................................................................................................... 2. ..................................................................................................................... (2) (Total 8 marks) Q21. Fluorine is a very useful element. It is placed in group 7 of the Periodic Table. Use your knowledge of the elements in group 7 to help you answer these questions. You may find that information in the Data Sheet may help you with this question. (a) Name another element in group 7 of the Periodic Table. Page 27 ..................................................................................................................................... (1) (b) Cylinders filled with fluorine molecules are commercially available. What would you expect the formula of a fluorine molecule to be? ..................................................................................................................................... (1) (c) Fluoride ions are added to drinking water to help prevent tooth decay. What is the charge on fluoride ions in the water? ..................................................................................................................................... (1) (d) Fluorine reacts with the non-metal sulphur to make sulphur hexafluoride (SF6). (i) What type of bonding would you expect in sulphur hexafluoride? .......................................................................................................................... (1) (ii) Explain the reason for your answer to part (i). .......................................................................................................................... .......................................................................................................................... .......................................................................................................................... (1) (Total 5 marks) Q22. The salt sodium hydrogen phosphate (Na2HPO4) is used as a softening agent in processed cheese. Page 28 It can be made by reacting phosphoric acid (H3PO4) with an alkali. (a) Complete the name of an alkali that could react with phosphoric acid to make sodium hydrogen phosphate. ....................................... hydroxide (1) (b) What is the name given to a reaction in which an acid reacts with an alkali to make a salt? ..................................................................................................................................... (1) (c) How would the pH change when alkali is added to the phosphoric acid solution? ..................................................................................................................................... ..................................................................................................................................... (1) (d) What ions are present when any acid is dissolved in water? ..................................................................................................................................... (1) (e) What ions are present when any alkali is dissolved in water? ..................................................................................................................................... (1) Page 29 (f) Write a chemical equation for the reaction which takes place between the ions you have named in (e) and (f). ..................................................................................................................................... (1) (Total 6 marks) Q23. The word equation below shows a reaction used in an industrial process. chromium oxide + aluminium → chromium + aluminium oxide The reaction is highly exothermic. (a) What is an exothermic reaction? ..................................................................................................................................... ..................................................................................................................................... (2) (b) Name the products of this reaction. ..................................................................................................................................... (1) (c) In the reaction one substance is reduced. (i) Name the substance which is reduced. .......................................................................................................................... (1) (ii) What happens to the substance when it is reduced? .......................................................................................................................... .......................................................................................................................... (1) (Total 5 marks) Page 30 Q24. Calcium carbonate reacts with dilute hydrochloric acid as shown in the equation below. CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g) The rate at which this reaction takes place can be studied by measuring the amount of carbon dioxide gas produced. The graph below shows the results of four experiments, 1 to 4. In each experiment the amount of calcium carbonate, the volume of acid and the concentration of the acid were kept the same but the temperature of the acid was changed each time. The calcium carbonate was in the form of small lumps of marble. (a) Apart from altering the temperature, suggest two ways in which the reaction of calcium carbonate and hydrochloric acid could be speeded up. 1. ................................................................................................................................ 2. ................................................................................................................................ (2) (b) Which graph, 1 to 4, shows the results of the experiment in which the acid had the highest temperature? Page 31 Experiment ................................................ Explain fully how you know. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (2) (c) (i) In experiment 2, how does the rate of reaction after one minute compare with the rate of reaction after two minutes? ........................................................................................................................... ........................................................................................................................... (1) (ii) Explain, as fully as you can, why the reaction rate changes during experiment 2. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (2) (Total 7 marks) Q25. This question is about sodium chloride (common salt) which is an important chemical. Sodium chloride can be made by burning sodium in chlorine gas. Page 32 (a) Balance the symbol equation for the reaction of sodium with chlorine. Na(s) + Cl2(g) → NaCl(s) (1) (b) (i) Complete the diagrams below to show the electronic structures of a sodium and a chlorine atom. (Atomic number of sodium = 11 and chlorine = 17.) (3) (ii) When sodium reacts with chlorine the sodium atoms are changed into sodium ions (Na+) and the chlorine atoms are changed into chlorine ions (Cl–). Explain how: 1. a sodium atom changes into a sodium ion; ........................................................................................................................... ........................................................................................................................... Page 33 (2) 2. a chlorine atom changes into a chloride ion. ........................................................................................................................... ........................................................................................................................... (2) (c) The element potassium is in the same group of the Periodic Table as sodium. Potassium reacts with chlorine to make potassium chloride which is sometimes used instead of common salt in cooking. (i) Predict the formula of potassium chloride. ........................................................................................................................... (1) By reference to the electronic structures of potassium and sodium explain: (ii) Why the reaction of potassium with chlorine is similar to the reaction of sodium with chlorine. ........................................................................................................................... ........................................................................................................................... (1) (d) The electrolysis of sodium chloride solution is an important industrial process. The diagrams below show two experiments set up during an investigation of the electrolysis of sodium chloride. Page 34 (i) What would be the reading on the ammeter in experiment 1? .................................................... A (ii) Explain your answer. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (3) (e) The equations below show the reactions which take place in experiment 2. (i) H2O(1) → H+(aq) 2H+(aq) + 2e– → H2(g) 2Cl–(aq) – 2e– → Cl2(g) + OH– (aq) Which substance provides hydrogen ions? ........................................................................................................................... (1) (ii) Name the product formed at: (A) the positive electrode; ........................................................................................................................... (B) the negative electrode. ........................................................................................................................... (1) (Total 15 marks) Page 35 Q26. Ammonia and nitric acid are both important chemicals. Nitric acid is made from ammonia. The charts below show substances made from ammonia and nitric acid. (a) Use the charts to help you answer these questions. (i) What is the main use of both ammonia and nitric acid? .......................................................................................................................... (1) (ii) Work out the percentage of ammonia used to make nitric acid. Percentage = ........................ % (1) (iii) 100 million tonnes of ammonia are made in the world each year. How much of this ammonia is used to make nylon? .......................... million tonnes (1) (b) The word equations below show how nitric acid is made. 1. nitrogen + hydrogen → ammonia 2. ammonia + oxygen → nitrogen monoxide + water Page 36 3. nitrogen monoxide + oxygen → nitrogen dioxide 4. nitrogen dioxide + water → nitric acid Use the word equations to help you answer these questions. (i) From which two elements is ammonia made? ......................................................... and ......................................................... (1) (ii) Name two of the raw materials needed to make nitric acid. ......................................................... and ......................................................... (2) (c) A large amount of nitric acid is reacted with ammonia to make a fertiliser. nitric acid + ammonia → fertiliser (i) The reaction is a neutralisation reaction. What type of chemical must ammonia be? ........................................................................................................................... (1) (ii) Complete the chemical name for the fertiliser made from ammonia and nitric acid. ammonium .................................................... (1) (iii) The reaction of nitric acid with ammonia is exothermic. Name the piece of equipment you could put into the solution to prove that the reaction is exothermic. ........................................................................................................................... (1) (Total 9 marks) Page 37 Q27. An electric current was passed through dilute sulphuric acid. The apparatus used is shown. Oxygen was formed at the anode. (a) What name is given to solutions which decompose when electricity is passed through them? ..................................................................................................................................... (1) (b) The ionic equation for the reaction at the anode is: 4OH– → 2H2O + O2 + 4e– Explain this type of reaction. ..................................................................................................................................... ..................................................................................................................................... (2) (c) Write a balanced ionic equation for the reaction at the cathode. ..................................................................................................................................... (2) Page 38 (d) What happens to the concentration of the sulphuric acid as the electricity is passed through it? Explain your answer. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (3) (Total 8 marks) Q28. This article appeared in a newspaper. (a) The balanced chemical equation shows the reaction between steel and hydrochloric acid. Fe(s) + 2HCl(aq) → FeCl2(aq) + H2(g) (i) Which metal in steel reacted with the hydrochloric acid? ........................................................................................................................... (1) (ii) The gas released was described as explosive. Explain why. ........................................................................................................................... ........................................................................................................................... Page 39 ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (3) (b) In the factory hydrogen chloride is manufactured by reacting hydrogen with chlorine. Hydrochloric acid is formed when hydrogen chloride forms a solution in water. (i) Water was sprayed on the steel and hydrochloric acid. This slowed the rate of reaction. Explain why. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (2) (ii) It would have been better to neutralise the acid with an alkali rather than to just add water. Hydrochloric acid can be neutralised by reaction with sodium hydroxide. Complete the ionic equation for the neutralisation reaction. (aq) + (aq) → H2O(l) (2) (iii) In the factory the acid leak was neutralised with slaked lime, Ca(OH)2, and not sodium hydroxide, NaOH. Suggest why. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (2) (Total 10 marks) Page 40 Q29. Brine, a solution containing sodium chloride in water, can be used to manufacture chlorine, hydrogen and sodium hydroxide. A student sets up a simplified model of the industrial cell. (a) The electron arrangements of some atoms are shown here. (i) H 1 O 2.6 Na 2.8.1 C1 2.8.7 Use the relevant electron arrangements to describe the bonding in water. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (2) (ii) Use the relevant electron arrangements to describe the bonding in sodium chloride. Page 41 ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (3) (b) Use the atomic structures of isotopes. and to explain the meaning of the term ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (3) (Total 8 marks) Q30. Titanium is a transition metal used as pins and plates to support badly broken bones. Titanium is extracted from an ore that contains the mineral titanium oxide. This oxide is converted into titanium chloride. Titanium chloride is heated with sodium to form titanium metal. This reaction takes place in an atmosphere of a noble gas, such as argon. 4Na(s) + TiCl4(l) → Ti(s) + 4NaCl(s) Calculate the mass of titanium that can be extracted from 570 kg of titanium chloride. Relative atomic masses: Cl 35.5; Ti 48. ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... Page 42 Mass of titanium = ............................ kg (Total 3 marks) Q31. Calcium tablets are taken to build and maintain strong bones and teeth. (a) These tablets react with hydrochloric acid in the stomach. CaCO3( (i) ) + 2HCl(aq) → CaCl2( ) + H2O( Add all these missing state symbols equation. ) + CO2( ) to the balanced chemical (2) (ii) The calcium salt that is formed is absorbed during digestion. What is the name of the calcium salt? ........................................................................................................................... ........................................................................................................................... (1) (b) The volume of carbon dioxide produced by one calcium tablet in the stomach can be found as shown. Page 43 The volume of carbon dioxide was recorded every 30 seconds until the reaction stopped. Time in seconds 0 30 60 90 120 150 180 210 240 Volume of gas in cm3 0 24 36 46 52 56 59 60 60 (i) Complete the graph of these results. (3) (ii) Describe one way in which this reaction can be made to go faster. ........................................................................................................................... Page 44 ........................................................................................................................... (1) (iii) A calculation, using the mass of this tablet, showed that 80 cm3 of carbon dioxide would be produced if the tablet was pure calcium carbonate. What do the results show about the purity of the tablet? Explain your answer by calculating the purity of this tablet. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (3) (Total 10 marks) Q32. Follow the steps to find the percentage of iron in iron oxide. Relative atomic masses: O 16; Fe 56. (i) Step 1 Calculate the relative formula mass of iron oxide, Fe2O3. ..................................................................................................................................... ..................................................................................................................................... (1) (ii) Step 2 Calculate the total relative mass of just the iron atoms in the formula, Fe2O3. ..................................................................................................................................... (1) (iii) Step 3 Page 45 Calculate the percentage (%) of iron in the iron oxide, Fe2O3. ..................................................................................................................................... ..................................................................................................................................... Percentage of iron ................................. % (1) (Total 3 marks) Q33. Many everyday substances can be classified as acids, bases or salts. For example, car batteries contain sulphuric acid, oven cleaners contain sodium hydroxide and table salt contains sodium chloride. (a) A solution of each of these substances was tested with universal indicator. Solution Colour of universal indicator Sulphuric acid (H2SO4) red Sodium hydroxide (NaOH) purple Sodium chloride (NaCl) green (i) Explain how these universal indicator colours and the corresponding pH values could be used to identify each of these solutions. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (3) (ii) Name and give the formula of the ion which causes the solution to be acidic. Name of ion .................................................................................................. Page 46 Formula of ion ................................................................................................. (2) (b) Sodium chloride can be made by reacting sodium hydroxide with hydrochloric acid in the presence of an indicator. (i) What is the name of this type of reaction? .......................................................................................................................... (1) (ii) Write a balanced chemical equation for this reaction. ..............(aq) + ..............(aq) → ..............(aq) + ..............(l) (2) (c) The atomic number for sodium is 11 and for chlorine is 17. (i) Complete the diagrams to show the electron arrangements for a sodium atom and a chlorine atom. (2) (ii) These atoms form different particles by one electron transferring from the sodium atom to the chlorine atom. What is the name given to the particles formed? ........................................................................................................................... (1) (iii) Why do these sodium and chloride particles bond? Page 47 ........................................................................................................................... ........................................................................................................................... (1) (d) Sodium chloride solution is electrolysed to form three products, hydrogen, chlorine and sodium hydroxide. Describe how each of these products are formed. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (3) (Total 15 marks) Q34. The diagrams show three isotopes of potassium. Page 48 (i) In what way does the atomic structure show you that they are all atoms? ..................................................................................................................................... ..................................................................................................................................... (1) (ii) Explain why these three atoms are called isotopes of potassium. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (3) (Total 4 marks) Q35. An investigation into the electrolyte copper sulphate solution was carried out as shown. Page 49 (a) What does electrolyte mean? ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (2) (b) These were the observations. (i) Negative electrode solid formed Positive electrode gas given off Name the solid formed. .......................................................................................................................... (1) (ii) Name the gas given off. .......................................................................................................................... (1) (c) How could a sample of gas be collected at the positive electrode? .................................................................................................................................... .................................................................................................................................... (2) Page 50 (d) Suggest why the blue colour of copper sulphate becomes paler during the investigation. .................................................................................................................................... .................................................................................................................................... (2) (Total 8 marks) Q36. This question is about rates of reaction. (a) Hydrogen peroxide (H2O2) decomposes very slowly at room temperature. (i) Complete the balanced chemical equation for this reaction by writing in the formula of the missing product. 2H2O2 → 2 ............... + O2 (1) (ii) The decomposition is much faster if manganese oxide is mixed with the hydrogen peroxide. Complete the sentence. Manganese oxide acts as a ................................... for decomposition of hydrogen peroxide. (1) (b) In an experiment 1g of manganese oxide was mixed with 50 cm3 of hydrogen peroxide solution. The results show the volume of oxygen collected during six minutes. Page 51 Time in minutes 0 1 2 3 4 5 6 Volume of oxygen in cm3 0 34.5 47.5 54.5 58.5 60.0 60.0 (i) Draw a graph of these results. (3) (ii) How long did it take for the decomposition to stop? ........................................................................................................................... (1) (iii) Why did the decomposition stop? ........................................................................................................................... (1) (c) In a second experiment water had been added to the hydrogen peroxide solution. Page 52 Again 50 cm3 of this hydrogen peroxide solution was mixed with 1g of manganese oxide. (i) For this second experiment, sketch, on the same grid, a graph line you would expect to get. (2) (ii) In this second experiment, why would the rate of reaction be different to the first experiment? ........................................................................................................................... (1) (Total 10 marks) Q37. Sodium reacts with chlorine to form the compound sodium chloride. 2Na + Cl2 → 2NaCl Describe, in terms of electron arrangement, the type of bonding in: (i) a molecule of chlorine; .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... (3) (ii) the compound sodium chloride. .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... Page 53 .................................................................................................................................... .................................................................................................................................... (4) (Total 7 marks) Q38. Limestone is a useful mineral. Every day, large amounts of limestone are heated in limekilns to produce lime. Lime is used in the manufacture of iron, cement and glass and for neutralising acidic soils. CaCO3 (i) CaO + CO2 The decomposition of limestone is a reversible reaction. Explain what this means. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (2) (ii) Calculate the mass of lime, CaO, that would be produced from 250 tonnes of limestone, CaCO3. Page 54 Relative atomic masses: C 12; O 16; Ca 40. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... Mass of lime = ........................................ tonnes (3) (Total 5 marks) Q39. Acids and bases are commonly found around the home. (a) Baking powder contains sodium hydrogencarbonate mixed with an acid. (i) When water is added, the baking powder releases carbon dioxide. How could you test the gas to show that it is carbon dioxide? Test .................................................................................................................. Result of test .................................................................................................... (2) (ii) Complete and balance the chemical equation for the reaction of sodium hydrogencarbonate with sulphuric acid. NaHCO3 + H2SO4 → ....................... + ........................ + ................ .. (2) (b) Indigestion tablets contain bases which cure indigestion by neutralising excess stomach acid. Page 55 (i) One type of indigestion tablet contains magnesium hydroxide. This base neutralises stomach acid as shown by the balanced chemical equation. Mg(OH)2 + 2HCl → MgCl2 + 2H2O Write a balanced ionic equation for the neutralisation reaction. .......................................................................................................................... (2) (ii) How does the pH in the stomach change after taking the tablets? .......................................................................................................................... (1) (c) Ammonium sulphate is used as a lawn fertiliser. Using ammonia solution, describe how you would make the fertiliser ammonium sulphate. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... Page 56 ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (3) (Total 10 marks) Q40. A student investigated the electrolysis of lead bromide. Lead bromide was placed in the tube and the circuit was switched on. The light bulb did not light up. The tube was heated and soon the bulb lit up. The observations are shown in the table. (a) Positive electrode Negative electrode red-brown gas silver liquid What is meant by electrolysis? .................................................................................................................................... (2) Page 57 (b) Why did the lead bromide conduct electricity when the tube was heated? .................................................................................................................................... (1) (c) Name the substances formed at the: positive electrode; ..................................................................................................... negative electrode. .................................................................................................... (2) (d) Suggest one safety precaution that should be taken during this investigation. .................................................................................................................................... (1) (Total 6 marks) Q41. Mountaineers can warm their food in self-heating, sealed containers. (a) The water is allowed to react with the lime. The heat from the reaction warms the food. What type of reaction causes a rise in temperature? .................................................................................................................................... (1) Page 58 (b) Some students investigated the effect of adding different sized lumps of lime to water. The results of their investigation are shown. Temperature in °C Time in minutes Large lumps of lime Small lumps of lime Powdered lime 0 18 18 18 1 19 20 28 2 21 23 43 3 24 27 63 4 28 32 88 5 33 38 100 What do these results show? Give an explanation for your answer. .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... (2) (c) Suggest and explain one disadvantage of using powdered lime to heat food. .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... (2) (Total 5 marks) Q42. A student tried to make some magnesium sulphate. Excess magnesium was added to dilute sulphuric acid. During this reaction fizzing was observed due to the production of a gas. Page 59 (i) Complete and balance the chemical equation for this reaction. ..................... + H2SO4 → ....................... + ........................ (3) (ii) At the end of the reaction the solution remaining was filtered. Why was the solution filtered? ..................................................................................................................................... (1) (iii) The filtered solution was left in a warm place. Explain why the filtered solution was left in a warm place. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (2) (Total 6 marks) Q43. A student studied the effect of temperature on the rate of reaction between hydrochloric acid and sodium thiosulphate. Page 60 • The student mixed 50 cm3 of a sodium thiosulphate solution and 5 cm3 of hydrochloric acid in a flask. • The flask was placed over a cross. • The student timed how long after mixing the cross could no longer be seen. (a) (i) Balance the chemical equation for this reaction. Na2S2O3(aq) + HCl(aq) → NaCl(aq) + H2O(l) + SO2(g) + S(s) (1) (ii) What causes the cross to be seen no longer? .......................................................................................................................... (1) (b) A graph of the results is shown. Page 61 (i) What effect does temperature have on the rate of this reaction? .......................................................................................................................... (1) (ii) Explain why temperature has this effect on the rate of reaction. .......................................................................................................................... .......................................................................................................................... .......................................................................................................................... .......................................................................................................................... (2) (Total 5 marks) Q44. As the world population increases there is a greater demand for fertilisers. Page 62 (a) Explain what fertilisers are used for. .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... (2) (b) The amount of nitrogen in a fertiliser is important. (i) How many nitrogen atoms are there in the formula, NH4NO3? ......................................................................................................................... (1) (ii) Work out the relative formula mass of ammonium nitrate, NH4NO3. Relative atomic masses: H 1; N 14; O 16. ........................................................................................................................... ........................................................................................................................... Relative formula mass of ammonium nitrate = ............................... (1) (Total 4 marks) Page 63 Q45. Uranium metal can be produced by reacting uranium hexafluoride with calcium. UF6 + 3Ca → 3CaF2 + U (a) Describe how calcium and fluorine bond together to form calcium fluoride. The electron arrangement of each atom is shown. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (5) (b) Uranium has two main isotopes, what is meant by the word isotope. and . Use these as examples to explain ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (4) Page 64 (c) At the start of a reaction there was 174.5 g of uranium hexafluoride, UF6. Relative atomic masses: F 19; U 235 (i) Calculate the relative formula mass of uranium hexafluoride, UF6. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... Relative formula mass UF6 = .................................... g (1) (ii) Calculate the mass of uranium that would be produced from 134.5 g of uranium hexafluoride. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... Mass of uranium = .................................. g (2) (Total 12 marks) Q46. Sodium chloride solution is a useful raw material for the manufacture of other substances. Page 65 (i) What is the name of the process shown? ..................................................................................................................................... (1) (ii) Chloride ions lose electrons at the positive electrode. What is the name of this type of reaction? ..................................................................................................................................... (1) (iii) The solution formed at X is alkaline. What causes this solution to be alkaline? ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (2) (iv) Give a balanced ionic equation for the formation of hydrogen gas at the negative electrode. ..................................................................................................................................... (3) (Total 7 marks) Page 66 Q47. (a) Two experiments were set up as shown. (i) Give two observations which would be seen only in Experiment D. 1 ....................................................................................................................... 2 ....................................................................................................................... (2) (ii) Explain why in Experiment C no changes would be seen. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (2) (b) Another electrolysis experiment used an aqueous solution of copper chloride. Page 67 (i) What does electrolysis mean? ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (2) (ii) Name the gas A and the deposit B. Gas A ................................................................................................................ Deposit B .......................................................................................................... (2) (c) Give one industrial use of electrolysis. ..................................................................................................................................... (1) (Total 9 marks) Q48. Hydrogen peroxide, H2O2, is often used as a bleach. It decomposes forming water and oxygen. (a) (i) Write the balanced chemical equation for the decomposition of hydrogen peroxide. Page 68 .......................................................................................................................... (3) (ii) Give a test for oxygen. Test .................................................................................................................. Result of test .................................................................................................... .......................................................................................................................... (2) (b) The rate of decomposition of hydrogen peroxide at room temperature is very slow. Manganese oxide is a catalyst which can be used to speed up the decomposition. Complete the sentence. A catalyst is a substance which speeds up a chemical reaction. At the end of the reaction, the catalyst is ............................................................................................... (1) (c) Two experiments were carried out to test if the amount of manganese oxide, MnO2 affected the rate at which the hydrogen peroxide decomposed. (i) Complete the diagram to show how you could measure the volume of oxygen formed during the decomposition. (2) (ii) The results are shown in the table. Time in minutes 0 0.5 1 1.5 2 2.5 3 3.5 Volume of gas in cm3 0 29 55 77 98 116 132 144 Page 69 using 0.25 g MnO2 Volume of gas in cm3 using 2.5 g MnO2 0 45 84 118 145 162 174 182 Draw a graph of these results. The graph for 0.25 g MnO2 has been drawn for you. (3) (iii) Explain why the slopes of the graphs become less steep during the reaction. ......................................................................................................................... ......................................................................................................................... ......................................................................................................................... (2) (iv) The same volume and concentration of hydrogen peroxide solution was used for both experiments. What two other factors must be kept the same to make it a fair test? 1 ....................................................................................................................... .......................................................................................................................... Page 70 2 ....................................................................................................................... .......................................................................................................................... (2) (Total 15 marks) Q49. The chemical equation for the formation of iron is: Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g) Calculate the relative formula mass of iron oxide, Fe2O3. Relative atomic masses: O 16; Fe 56. ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... Relative formula mass Fe2O3 = ................................ (Total 2 marks) Q50. Bordeaux Mixture controls some fungal infections on plants. A student wanted to make some Bordeaux Mixture. Page 71 (a) The student knew that calcium oxide could be made by heating limestone. Limestone contains calcium carbonate, CaCO3. (i) Write the word equation for this reaction. ........................................................................................................................... (1) (ii) What type of reaction is this? ........................................................................................................................... (1) (b) The student knew that copper sulphate, CuSO4, could be made by the following general reaction. acid + base → salt + water (i) What type of reaction is this? ........................................................................................................................... (1) (ii) The base used is copper oxide. Name and give the chemical formula of the acid used. Name ................................................................................................................ Chemical formula ............................................................................................. (2) (c) The student wrote about how the copper sulphate was made. “Some of the acid was warmed. Copper oxide was added. The mixture was stirred. More copper oxide was added until no more would react. The mixture was then filtered.” (i) Why was the acid warmed? ........................................................................................................................... ........................................................................................................................... (1) Page 72 (ii) Copper oxide was added until no more would react. Explain why. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (2) (iii) The filtration apparatus is shown. Describe and explain what happens as the mixture is filtered. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (2) (Total 10 marks) Q51. There is molten rock below the Earth’s solid outer crust. The rock remains molten because the radioactive decay of isotopes such as uranium, thorium and potassium releases heat energy. (i) Explain how this released heat energy is thought to cause the recycling of rocks. ..................................................................................................................................... ..................................................................................................................................... Page 73 ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (4) (ii) Two isotopes of potassium are shown. Explain what is meant by isotopes. You must include numbers of electrons, neutrons and protons in your explanation. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (4) (Total 8 marks) Q52. Millions of years ago the Earth formed as a giant ball of molten rock. The outer surface cooled forming a thin, solid outer crust. Volcanic activity on the surface produced an atmosphere containing the compounds carbon dioxide, ammonia, methane and water vapour. Describe the bonding in any one of these compounds. You must include electronic structures in your explanation. ............................................................................................................................................... Page 74 ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... (Total 4 marks) Q53. (a) Indigestion tablets called antacids can be taken to react with excess hydrochloric acid in the stomach. A student investigated two different antacid tablets labelled X and Y. (i) Both tablets, X and Y, contained calcium carbonate. Give the chemical symbol for each of the three elements in calcium carbonate. .......................................................................................................................... .......................................................................................................................... (3) (ii) Name the gas formed when calcium carbonate reacts with hydrochloric acid. .......................................................................................................................... (1) (b) The student first reacted tablet X and then tablet Y, with 100 cm3 of a hydrochloric acid solution. The student measured the volume of gas produced during the first five minutes. The results are shown in the table. Time in minutes 0 1 2 3 4 5 Volume of gas in cm3 Tablet X 0 38 48 48 48 48 Volume of gas in cm3 Tablet Y 0 31 54 67 72 72 Page 75 (i) Draw a graph of the results for tablet Y. (A graph of the results for tablet X has been drawn for you.) (3) (ii) Tablet X contains less calcium carbonate than tablet Y. How do the results show this? .......................................................................................................................... .......................................................................................................................... (1) (iii) Explain why the rate of reaction slows down for both tablets. .......................................................................................................................... .......................................................................................................................... .......................................................................................................................... (2) (Total 10 marks) Page 76 Q54. (a) The diagram shows one way of making crystals of copper sulphate. (i) Why was the solution filtered? .......................................................................................................................... (1) (ii) How could you make the crystals form faster from the copper sulphate solution? .......................................................................................................................... (1) (iii) The chemical equation is shown for this reaction. CuO(s) + H2SO4(aq) → CuSO4(aq) + H2O(l) In the chemical equation what does (aq) mean? .......................................................................................................................... (1) (b) Blue copper sulphate crystals go white when warmed. How could you use the white copper sulphate as a test for water? Page 77 .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... (2) (Total 5 marks) Q55. Petrol is a mixture of hydrocarbons such as octane, C8H18 When petrol is burned in a car engine, a large amount of carbon dioxide is produced. This car uses 114 g of petrol to travel one mile. Calculate the mass of carbon dioxide produced when this car travels one mile. Assume that petrol is octane and that combustion is complete. (Relative atomic masses: H = 1; C = 12; O = 16) The combustion of octane can be represented by this equation. C8H18 + 12 O2 → 8CO2 + 9H2O ............................................................................................................................................... ............................................................................................................................................... Page 78 ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... Mass of carbon dioxide = ........................ g (Total 3 marks) Q56. Explain, in terms of ions and molecules, what happens when any acid reacts with any alkali. ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... (Total 3 marks) Q57. The information in the box is about the preparation of copper sulphate crystals. Step 1 Add a small amount of black copper oxide to some hot dilute sulphuric acid, and stir. Step 2 Keep adding copper oxide until it is in excess. Step 3 Remove the excess copper oxide to leave blue copper sulphate solution. Step 4 Evaporate the copper sulphate solution until it is saturated. Step 5 Leave the saturated solution of copper sulphate to cool. Blue copper sulphate form on cooling. Page 79 crystals Step 6 Remove the crystals from the solution remaining. Step 7 Dry the blue crystals on a piece of filter paper. (i) Suggest a reason for using excess copper oxide in Step 2. ..................................................................................................................................... ..................................................................................................................................... (1) (ii) Suggest how the excess copper oxide can be removed from the solution in Step 3. ..................................................................................................................................... ..................................................................................................................................... (1) (iii) What is meant by the term saturated solution? ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (2) (iv) Why do crystals form when a hot saturated solution cools? ..................................................................................................................................... ..................................................................................................................................... (1) (v) Suggest why the blue crystals are dried in Step 7 using filter paper instead of by heating. ..................................................................................................................................... ..................................................................................................................................... (1) (Total 6 marks) Page 80 Q58. Ammonia (NH3) is an important chemical which is used to make fertilisers. Ammonia is made from nitrogen and hydrogen, (a) The diagrams represent the electron arrangements in atoms of nitrogen and hydrogen. Complete the diagram showing the arrangement of electrons in a molecule of ammonia. (1) (b) Name the type of bonding which holds the nitrogen and hydrogen atoms together in an ammonia molecule. ..................................................................................................................................... (1) (Total 2 marks) Q59. A student studied the reaction between dilute hydrochloric acid and an excess of calcium carbonate. calcium carbonate + hydrochloric acid → calcium chloride + water + carbon dioxide Page 81 The student measured the volume of carbon dioxide produced in the experiment. The results are shown on the graph. (a) After how many minutes had all the acid been used up? ............................................ minutes (1) (b) The student wrote this conclusion for the experiment: ‘The reaction gets slower and slower as the time increases.’ Explain why the reaction gets slower. Your answer should be in terms of particles. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (2) (c) A second experiment was carried out at a higher temperature. All other factors were Page 82 the same. Draw a line on the graph above to show the results that you would expect. (2) (Total 5 marks) Q60. (a) The flow diagram shows the stages in the production of nitric acid. Give the names of the compounds labelled as A, B and C on the flow diagram. Choose names from the box. ammonia nitrogen nitrogen dioxide nitrogen monoxide A ........................................................................ B......................................................................... C ........................................................................ (3) Page 83 (b) Use the flow diagram to help you name two raw materials used to make nitric acid. ............................................................... and ............................................................... (2) (c) Reaction 1 uses a catalyst. (i) How does a catalyst help this reaction? ........................................................................................................................... (1) (ii) Draw a ring around the name of the catalyst used in reaction 1. copper iron platinum vanadium (1) (Total 7 marks) Q61. A student investigated some instant soup. (a) Instant soup contains a food additive which has the formula: NaH2PO4 Give the names of all the elements in this compound. The periodic table on the Data Sheet may help you to answer this question. ..................................................................................................................................... ..................................................................................................................................... (2) (b) The student investigated the reaction which takes place when soup powder is added to cold water. The student thought that the reaction might be exothermic. (i) What is meant by the term exothermic reaction? ........................................................................................................................... ........................................................................................................................... (2) Page 84 (ii) Describe an experiment that the student could do to prove that this reaction is exothermic. To gain full marks in this question you should write your ideas in good English. Put them into a sensible order and use the correct scientific words. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (4) (Total 8 marks) Q62. Salts can be prepared by the reaction of acids with alkalis. (a) (i) The reactions of acids with alkalis can be represented by the equation below. Choose a substance from the box to complete the equation. carbon dioxide hydrogen oxygen water acid + alkali → salt + .......................................................... (1) (ii) Draw a ring around the word which best describes the reaction. displacement neutralisation oxidation reduction (1) (b) Sodium sulphate is an important salt. The table gives a list of some substances. Page 85 Put a tick ( ) next to the names of the acid and the alkali that would react to make sodium sulphate. Substances ( ) Hydrochloric acid Nitric acid Potassium sulphate Sodium hydroxide Sodium nitrate Sulphuric acid (2) (Total 4 marks) Q63. Silicon is an important element used in the electronics industry. (a) Silicon can be made by heating a mixture of sand (silicon dioxide) with magnesium powder. The equation for this reaction is shown below. SiO2 (s)+ 2Mg (s) → 2MgO (s) + Si (s) Calculate the mass of silicon dioxide needed to make 1 g of silicon. Relative atomic masses: O = 16; Si = 28 ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... Mass = ........................................................g (3) (b) The resulting mixture of magnesium oxide and silicon is added to a beaker containing hydrochloric acid. The silicon is then filtered from the solution. Page 86 (i) The magnesium oxide reacts with the hydrochloric acid and forms magnesium chloride (MgCl2) solution and water. magnesium oxide + hydrochloric acid → magnesium chloride solution + water Write a balanced symbol equation for this reaction, including state symbols. .......................................................................................................................... (2) (ii) The gases produced are a mixture of several silicon hydrides. One of the gases produced in the reaction is the silicon hydride with the formula SiH4. The structure of this molecule is similar to methane, CH4. Draw a diagram to show the bonding in a molecule of SiH4. Represent the electrons as dots and crosses and only show the outer shell (energy level) electrons. (1) Page 87 (iii) A sample of a different silicon hydride was found to contain 1.4 g of silicon and 0.15 g of hydrogen. Calculate the formula of this silicon hydride. You must show all your working to gain full marks. Relative atomic masses: H = 1; Si = 28 ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (4) (iv) The silicon hydrides react immediately they come into contact with oxygen in the air. They burst into flames with a small explosion and give out energy. Which letter, A to H, best describes this reaction? Energy involved in breaking and forming bonds The energy released from forming new bonds is greater than the energy needed to break existing bonds Activation energy high low The energy needed to break existing bonds is greater than the energy released from forming new bonds high low Page 88 Rate of reaction Letter fast A slow B fast C slow D fast E slow F fast G slow H Letter ................... (1) (c) The structure of silicon is similar to the structure of diamond. Describe the structure of silicon and explain why it has a high melting point. You may draw a diagram if this helps. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (4) (Total 15 marks) Q64. The following passage is about the preparation of lead iodide, an insoluble salt. An excess of potassium iodide in solution was shaken with some lead nitrate solution in a test tube. The lead iodide precipitate was separated from the mixture and then washed several times with water. The lead iodide was dried and then placed in a bottle. (a) Suggest a reason why excess potassium iodide was used. ..................................................................................................................................... ..................................................................................................................................... (1) Page 89 (b) What word used in the passage shows that lead iodide is insoluble? .................................................................................................................................... (1) (c) Suggest how lead iodide can be separated from the mixture. .................................................................................................................................... .................................................................................................................................... (1) (d) Why was the lead iodide washed with water? .................................................................................................................................... .................................................................................................................................... (1) (e) Suggest a method which could be used to dry this lead iodide. .................................................................................................................................... .................................................................................................................................... (1) (f) Lead compounds are toxic. Suggest a suitable safety precaution that should be taken when using toxic substances in laboratories. .................................................................................................................................... (1) (Total 6 marks) Q65. (a) A tin of red kidney beans contains calcium chloride as a firming agent. Page 90 Calcium chloride is an ionic compound which contains calcium ions (Ca2+) and chloride ions (Cl–). (i) The diagram on the left represents the electronic structure of a chlorine atom. Complete a similar diagram on the right to represent a chloride ion. (2) (ii) Explain how a calcium atom changes into a calcium ion which has a 2+ charge. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (2) (b) Cola drinks contain phosphoric acid, H3PO4. The two equations show how phosphoric acid can be made from phosphorus. Balance these two equations. (i) P4 + ........ O2 → P4O10 (1) (ii) P4O10 + ................ H2O → 4H3PO4 (1) Page 91 (Total 6 marks) Q66. Calcium carbonate tablets are used to treat people with calcium deficiency. (a) Calculate the relative formula mass (Mr)of calcium carbonate. Relative atomic masses: C = 12; O = 16; Ca = 40. ..................................................................................................................................... ..................................................................................................................................... Relative formula mass = .............................. (2) (b) Calculate the percentage of calcium in calcium carbonate, CaCO3. ..................................................................................................................................... ..................................................................................................................................... Percentage of calcium = .......................... % (2) (c) Calculate the mass of calcium in each tablet. ..................................................................................................................................... ..................................................................................................................................... Page 92 Mass of calcium = .................................... g (2) (d) An unwanted side effect of this medicine is that it can cause the patient to have ‘wind’ (too much gas in the intestine). The equation below represents the reaction between calcium carbonate and hydrochloric acid (the acid present in the stomach). CaCO3 (s) + 2HCl (aq) →CaCl2 (aq) + H2O (l) + CO2 (g) Suggest why the patient may suffer from ‘wind’. ..................................................................................................................................... ..................................................................................................................................... (1) (Total 7 marks) Q67. The electrolysis of sodium chloride solution is an important industrial process. The apparatus shown below can be used to show this electrolysis in the laboratory. (a) Name gas A. ............................................................................................................... (1) Page 93 (b) Chlorine is produced at the positive electrode. Describe and give the result of a chemical test to prove that the gas is chlorine. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (2) (c) Chloride ions move to the positive electrode. Explain why. ..................................................................................................................................... ..................................................................................................................................... (1) (d) A small quantity of chlorine is added to drinking water. Explain why. ..................................................................................................................................... ..................................................................................................................................... (1) (e) The solution around the negative electrode becomes alkaline. Name the ion which makes the solution alkaline. ..................................................................................................................................... ..................................................................................................................................... (1) (Total 6 marks) Q68. A student did two experiments using ammonium chloride. (a) In the first experiment the student heated a small amount of ammonium chloride in a test tube. Page 94 Two reactions take place in the test tube. Reaction 1 ammonium chloride → ammonia + hydrogen chloride (colourless gases) Reaction 2 ammonia + hydrogen chloride → ammonium chloride (i) Complete the sentences by crossing out the incorrect word in each box. Reaction 1 takes place at a high low temperature. Reaction 2 takes place at a high low temperature. (1) (ii) Draw a ring around the word which best describes reactions 1 and 2. combustion displacement oxidation reduction reversible (1) (iii) Suggest a reason for the mineral wool at the top of the test tube. ........................................................................................................................... ........................................................................................................................... Page 95 (1) (b) In the second experiment the student mixed a small amount of ammonium chloride with some water in a beaker. The temperature of the water was measured before and after adding the ammonium chloride. Temperature before adding the ammonium chloride 20°C Temperature after adding the ammonium chloride 16°C Draw a ring around the word which best describes the process which takes place. combustion displacement endothermic exothermic freezing (1) (Total 4 marks) Q69. Ammonium nitrate and potassium chloride are both salts. They can be made by neutralisation reactions. Choose substances from the box to complete the word equations for the formation of these two salts. ammonia potassium nitrate hydrochloric acid water nitric acid potassium hydroxide ammonia + ........................................ → ammonium nitrate + water .................................. + hydrochloric acid → potassium chloride + .......................... (Total 3 marks) Q70. This label was on a bottle of stain remover. Page 96 When ‘Simply Amazing’ is mixed with water a reaction takes place which produces bubbles of oxygen gas. (i) Suggest a method that you could use to measure how quickly this reaction takes place. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (2) (ii) Read the instructions on the label and then suggest how increasing the temperature of the water affects the rate of this reaction. ..................................................................................................................................... ..................................................................................................................................... (1) Page 97 (iii) Suggest one other way in which the rate of a reaction can be changed. ..................................................................................................................................... ..................................................................................................................................... (1) (Total 4 marks) Q71. This cake recipe is taken from a cookery book. Soda Cake • • • • Mix the flour and butter and add the sugar, currants and flavouring. Then add the beaten egg. Add a little milk with a teaspoonful of baking soda (sodium hydrogencarbonate) and mix it in well. Bake in a moderate oven for about 30 minutes. When sodium hydrogencarbonate is heated in an oven, it forms carbon dioxide gas. 2 NaHCO3 Na2CO3 + H2O + CO2 A teaspoonful of baking soda contains a mass of 11 g of sodium hydrogencarbonate. Calculate the mass of carbon dioxide that could be made from 11 g of sodium hydrogencarbonate. Show clearly how you work out your final answer. Relative atomic masses: H = 1; C = 12; O = 16; Na = 23. ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... Mass of carbon dioxide = ............................................... g (Total 3 marks) Page 98 Page 99 M1. Factor 1 heating the solution / heat / increasing temperature / candidates can gain one mark here for the idea of the water evaporating faster with increased heat (so heating the reactants faster). particles (of fat and sodium hydroxide) move faster (not vibration / not just move more) / more kinetic energy collide more often / more collisions have more energy when they collide / more successful collisions Factor 2 concentrated (solution of alkali) more (sodium hydroxide) particles (in a given volume) particles closer/ more crowded etc. more collisions / greater chance of successful collisions each for 1 mark Possible alternative answer size of fat pieces / small pieces of fat have larger surface area more collisions / greater chance of collisions [7] M2. (a) some electrons from outer shells (some electrons) free to move/mobile through whole structure/between atoms/sea of electrons hold atoms together for 1 mark each or positive ions in a sea of electrons (owtte) Page 100 2 marks atoms in regular structure/layers giant structure close packed credit diagrams – look for labels for 1 mark each any 4 4 (b) (i) electrons, free to move (reference to electrons) for 1 mark each 2 (ii) layers/atoms can slide over each other for 1 mark 1 (iii) free electrons hold atoms strongly together/strong forces of attraction/bonds (between atoms)/tight packing of atoms for 1 mark 1 [8] ## (a) (2) : (6) : (2) All 3 correct gains 2 marks 2 correct gains 1 mark 2 (b) no water present/moist air cannot enter/do not thoroughly mix/ must be in solution etc. for 1 mark 1 (c) (i) hydroxide (ion) / OH– for 1 mark 1 (ii) hydrogen (ion) / H+ for 1 mark 1 Page 101 (iii) water/H2O/hydrogen oxide for 1 mark 1 [6] M4. (a) Gas A = Chlorine / Cl2 not Cl and Gas B = Hydrogen / H2 not H for 1 mark Solution C = sodium hydroxide/NaOH/spent brine for 1 mark 2 (b) (i) 2, 2 for 1 mark (ii) 2, 2 for 1 mark 2 (c) water/H2O/hydrogen oxide not hydrogen hydroxide for 1 mark 1 (d) ions/positive ions/negative ions/cations/anions not charged particles/positive particles/negative particles not H+ / Cl-/Na+ / OHAllow hydrogen ions etc. not sulphate ions for 1 mark 1 [6] ## (a) covalent/description of covalent for 1 mark 1 Page 102 (b) forces/bonds between the molecules/particles (not atoms) are weak for 1 mark each 2 (c) non-flammable so it will not burn etc. extremely unreactive so it will not react with materials in the transformer, does not conduct electricity so it can insulate the transformer gas so it has freedom to move and insulate whole area for 1 mark each 3 [6] M6. (a) Group 2 / Alkaline Earth Metals for 1 mark 1 (b) (i) MgCl2/Mg2+ (Cl–)2 (or equation with correct answer) for 1 mark 1 (ii) ionic / electrovalent for 1 mark 1 [3] ## (a) potassium / K for 1 mark 1 (b) carbon dioxide / CO2 for 1 mark 1 Page 103 (c) losing electrons gaining electrons for 1 mark each 4 (d) (i) power supply, (not mains) beaker containing solution, (inert) electrodes and circuit ammeter or bulb/ (or see bubbling etc. at electrodes written by drawing) for 1 mark each 4 (ii) reading on ammeter/bulb lights / (solution) conducts (electricity) bubbling / gas produced hydrogen produced chlorine / oxygen produced ions move to electrodes (must be linked to ions move) negative ions move to the positive electrode and/or positive ions move to the negative electrode negative ions lose electrons and/or positive ions gain electrons any 3 for 1 mark each 3 [13] M8. (a) C16 H34 for 1 mark 1 (b) electron gains 1 mark but shared electrons gains 2 marks 2 [3] Page 104 M9. (a) 4 HCl / 2H2O, allow multiples or fractions if whole equation balances for 1 mark 1 (b) germanium tetrachloride + water = germanium oxide + hydrochloric acid If symbol equation given it must be correctly balanced Allow germanium for 1 mark 1 (c) to purify the germanium oxide/remove impurities/give in pure product/to make pure germanium for 1 mark 1 ensure complete reaction/reaction does not give a good yield not to increase efficiency/to purify germanium for 1 mark 1 (d) (i) remove oxygen/addition of hydrogen/gain up electrons allow remove oxygen molecules (ii) GeO2 = 73 + (2 × 16) = 105 mass of germanium = 525 × (73/105) = 365 g (or alternative methods) apply consequential marking for 1 mark each 3 (e) (i) germanium is shiny/lustrous conducts a small amount of electricity * germanium oxide reacts with hydrochloric acid (and) metal oxides react with acid metal oxides are basic metal oxides are reduced by hydrogen Page 105 Information must be taken from the passage. Apply the list principle if more than three answers are given. Assume the word ‘it’ refers to the metal. any 3 for 1 mark each 3 (ii) germanium is brittle germanium tetrachloride is a (volatile) liquid made of molecules germanium tetrachloride has covalent bonding or when two non-metals react they have covalent bonding GaC14/the salt of germanium undergiven hydrolysis/reacts with water germanium is not a good conductor of electricity* * conductivity mark can only be given once any 3 for 1 mark each 3 [13] M10. (a) 2, 8, 8, 1 for 1 mark 1 (b) for 1 mark Ignore symbol in middle but structure must be drawn NOT 2,7 If covalent; can score mark for changes but not for diagram Arrow showing electron transfer from metal atom to non-metal atom = 2 marks Page 106 If the ions are not identified then cannot score mark for changes 4 [5] M11. (a) (i) Na2CO3 or (Na+)2 CO32– must be completely correct for 1 mark 1 (ii) (1) decomposition of limestone or decomposition of coal or decomposition of sodium hydrogen carbonate (owtte.) allow equations even if not correctly balanced any 1 for 1 mark 1 (2) breakdown/split up not decomposed by heat for 1 mark each 2 (iii) carbon dioxide or ammonia [CO2] or [NH3] for 1 mark 1 (b) (i) zinc carbonate or zinc hydroxide allow formulae if completely correct for 1 mark 1 (ii) (zinc carbonate) is insoluble (in water) ((i) and (ii) are independent marks) for 1 mark 1 [7] M12. (a) (i) hydrogen/H2 Page 107 for 1 mark 1 (ii) i.e. 2Cl¯ -2e– →Cl2 for 1 mark 1 (iii) hydroxide or OH– for 1 mark 1 (iv) sodium hydroxide/caustic soda/NaOH/bleach/ chemical name of bleach for 1 mark 1 (b) (i) Na2CO3 or (Na+)2 CO32– for 1 mark 1 (ii) coal water/H2O limestone/CaCO3/calcium carbonate any one for 1 mark 1 (iii) calcium chloride/CaCl2/sodium hydrogen carbonate/NaHCO3 for 1 mark 1 (iv) decomposition/heating of limesstone decomposition/heating of coal decomposition/heating of sodium hydrogen carbonate any 1 for 1 mark 1 described change e.g. NaHCO3 → Na2 CO3 (Use judgement) breakdown (owtte.) by heat for 1 mark each 2 Page 108 (v) carbon dioxide/CO2 or ammonia/NH3 for 1 mark 1 (c) (i) zinc carbonate/ZnCO3/zinc hydroxide/Zn(OH)2 for 1 mark 1 (ii) It is insoluble zinc carbonate is insoluble in water for 1 mark 1 [13] M13. (a) 56g for 1 mark 1 (b) 44 tonnes for 1 mark 1 [2] M14. (a) 8 marks Particularly well structured answer with most points mentioned. 7-6 marks Well structured answer. The two metals will have been compared rather than simply listing advantages/disadvantages. Most of the advantages and disadvantages of each metal have been mentioned. 5-3 marks Some structure to the answer. An attempt to compare the metals by giving some advantages and disadvantages. Page 109 2-1 marks Little structure or attempt to compare. Marks gained by listing a few advantages or disadvantages. Advantages of Nickel: Relatively low cost which makes the sparking plugs cheaper to produce. Quite high melting point which is needed because the temperature in the engine is very high. Good conductor of electricity needed to carry electricity into combustion chamber to produce spark. Disadvantages of Nickel: Subject to corrosion in engine which means they only last a short time because nickel is higher in reactivity than platinum. Idea that this leads to reduced efficiency, unburnt petrol and air pollution. Advantages of Platinum: Less susceptible to corrosion (not corroded) because platinum is very low in reactivity. Idea that this improves efficiency and reduces pollution.Higher melting point than nickel to withstand the high temperatures in the combustion chamber. Last a lot longer than nickel electrodes due to low reactivity. (Sensible extension here could be longer service intervals etc.)Good conductor of electricity as for nickel. Extension here could be linked to the idea that the conductivity does not deteriorate as quickly as nickel.) Disadvantages of Platinum: Cost which will make the sparking plug more expensive. A good candidate might justify cost by longer life, better fuel consumption and less pollution. 8 (b) (i) giant structure/lattice/regular arrangements of atoms any for 1 mark Page 110 of atoms/of ions (provided free electrons mentioned) either for 1 mark delocalised or free electrons for 1 mark 3 (ii) electrons free/can move for 1 mark each 2 [13] M15. (a) (i) H+ + OH- → H2 O/H3O+ + OH- → 2H2 O for 1 mark 1 (ii) 1 point from e.g. smaller bits bigger surface area faster reaction dissolve faster more particles open to attack by acid any 1 for 1 mark 1 (iii) MgCO3 or MG2+CO32- or CO3 Mg for 1 mark 1 (b) (i) 2 HCl for 1 mark 1 (ii) aqueous/dissolved in water (not in solution) for 1 mark 1 Page 111 (iii) CO2/gas evolved/gas has mass for 1 mark 1 (c) (i) plotting points scales curve labelling axes including units for 1 mark each 4 (d) faster same final mass for 1 mark each 2 [12] M16. (i) neutralisation/acid base reaction for 1 mark 1 (ii) 17 (tonnes) give 80 (tonnes) (even if only in working) for 1 mark each 320 (tonnes) or alternative method) 3 marks for correct answer (if 17 and 80 not given allow 1 mark for correct answer using their figures) 3 [4] Page 112 M17. (a) (i) 45% for 1 mark 1 (ii) 126 000 (consequential on (i)) for 1 mark 1 (b) (i) Cl2 = 71 1 × 71/24 or correct mathematical attempt for 1 mark (If Cl2 wrong take figure given) for 1 mark = 2.96 kg gains 3 marks (or alternative methods) (if units not given - 3 marks. If units wrong - 2 marks) 3 (ii) any sensible eg. bleach/disinfectant/antiseptics/kill bacteria/ sterilise water/solvents/refrigerents/CFCs/PVC (not water treatment or warfare) for 1 mark 1 [6] M18. (i) 1 electrons for 1 mark (ii) covalent 1 for 1 mark (iii) made of small molecules: usually gas or liquid ) dependent on have low melting points ) having first Page 113 have low boiling points ) point above forces between molecules are weak any 1 for 1 mark 3 [5] M19. (a) 40 + 12 + (3 × 16) = 100 each for 1 mark 2 (b) Mr of CaO = 56 for 1 mark mass required = 60 × 100/56 for 2 marks = 107.1 for 1 mark 4 (c) (i) calcium hydroxide 1 (ii) solid 1 [8] M20. (a) (i) 14 electrons = gets 1 mark 2.8.4 = gets 2 marks 2 (ii) outer shell electrons 1 (iii) same number of electrons in outer shell 1 Page 114 (b) (1) shiny conducts electricity (2) oxide neutralises alkalis covalent bonds 4 [8] M21. (a) any (must be named) 1 (b) F2 1 (c) –/F– 1 (d) (i) covalent 1 (ii) made of molecules etc. type of bonding when non-metals react. 1 [5] M22. (a) sodium 1 (b) neutralisation 1 (c) increase/inc. number 1 (d) H+ 1 Page 115 (e) OH– (f) H+ + OH– → H2O 1 [6] M23. (a) gives out heat each for 1 mark 2 (b) chromium and aluminium oxide 1 (c) (i) chromium oxide 1 (ii) oxygen removed/gains electrons 1 [5] M24. (a) increase concentration of acid; increase surface area of solid or grind up the solid; add a catalyst any two for 1 mark each 2 (b) 1; it is the one that makes the gas fastest (steeper curve etc) (second part is dependant on first) for 1 mark each 2 Page 116 (c) (i) faster after one minute, slower after 2 minutes for 1 mark 1 (ii) the reactants get used up; so concentration decreases/less chance of collision for 1 mark each 2 [7] M25. (a) 2 2 multiples of ½ allowed for 1 mark 1 (b) (i) 2. 8. 1 and 2. 8. 7 gains 3 marks 1 mark for 2 electrons in each inner shell 1 mark for 8 electrons in each second shell 1 mark for 1 electron in sodium outer shell and 7 in chlorine outer shell 3 (ii) sodium atom loses; electron; chlorine atom gains; electron for 1 mark each inversion = 2 marks lose negative charge = 1 mark 4 (c) (i) KCl (accept 2KCl) for 1 mark 1 (ii) both have on electron in outer shell/same number of electrons/ lose same number of electrons in compound formation/ both lose one electron Page 117 for 1 mark 1 (d) 0 amps; the ions; cannot move in the solid solid Na chloride does not conduct for 1 mark each 3 (e) (i) water (H2O) for 1 mark 1 (ii) (1) chlorine; (2) hydrogen for 1 mark 1 [15] M26. (a) (i) fertilisers for 1 mark 1 (ii) 7 for 1 mark 1 (iii) 5 for 1 mark (ignore other units) 1 (b) (i) both nitrogen and hydrogen for 1 mark 1 (ii) two of: Page 118 nitrogen; hydrogen/methane/natural gas; oxygen/air; water; any fuel (allow symbols, do not allow nitrogen oxides) any two for 1 mark each 2 (c) (i) alkali/alkaline/base/basic for 1 mark 1 (ii) must be nitrate for 1 mark 1 (iii) thermometer or any other temperature measuring device for 1 mark 1 [9] M27. (a) electrolytes 1 (b) oxidation 1 electrons lost 1 (c) 2H+ + 2e– → H2 minus sign on e– not needed 2 (d) concentration increases 1 Page 119 OH– discharged from water / water decomposes 1 H+ concentration increases / H2 and O2 evolved 1 [8] M28. (a) (i) iron must be named do not accept Fe 1 (ii) hydrogen 1 and oxygen mixtures 1 burn rapidly 1 (b) (i) lowers concentration accept dilutes the acid do not accept cooling 1 less collisions (between particles) 1 (ii) H+ (aq) accept H3O+ only if 2 in front of H2O 1 OH (aq) if spectator ions correctly included on both sides, maximum = 1 mark 1 (iii) Ca(OH2) weak alkali accept NaOH strong alkali 1 Ca(OH)2 causes no problems Page 120 accept NaOH causes named problem (eg caustic or exothermic or burns or corrosive) 1 [10] M30. 144 accept TiCl4 = 190 for 1 mark accept another correct step in calculation eg 570/190 = 3 for 1 mark [3] M31. (a) (i) (s) (aq) (1) 2 or 3 correct 1 mark 1 correct 0 marks (g) 2 (ii) calcium chloride 1 (b) (i) points deduct 1 mark for each error to a maximum of 2 marks 2 line accept a single line ‘best fit’ curve accept reasonable attempt at curve 1 (ii) increase temperature or heat accept increase surface area or increase concentration or description 1 Page 121 (iii) 75% or ¾ not pure 1 mark only 60 cm3 (instead of 80 cm3 of gas) or × 100 1 mark 3 [10] M32. (i) 160 ignore units 1 (ii) 112 ignore units 1 (iii) 70 do not carry forward errors 1 [3] M33. (a) (i) H2SO4 or red (acidic) pH < 7 accept names of compounds accept correct use of acidic 1 NaOH or purple (alkaline) pH > 7 alkaline and neutral without any mention of pH for 1 mark only 1 NaCl or green (neutral) pH 7 ignore high or low pH 1 (ii) hydrogen (ion) Page 122 accept proton accept hydroxonium ion 1 H+ accept H3O+ for hydroxonium ion 1 (b) (i) neutralisation 1 (ii) NaOH + HCl ignore state symbols 1 NaCl + H2O ignore state symbols maximum of 1 mark if incorrectly balanced 1 (c) (i) sodium – 2 . 8 . 1 accept 2.8.1 written 1 chlorine – 2 . 8 . 7 accept 2.8.7 written 1 (ii) ion(s) 1 (iii) attraction between oppositely charged particles (ions) accept attraction between + and – particles (ions) accept electrostatic attraction 1 (d) chloride ions lose electrons to form chlorine Cl– – e– → Cl 1 hydrogen ions gain electrons to form hydrogen H+ + e– → H 1 sodium hydroxide remains in solution Page 123 Na + and OH– remain in solution to form sodium hydroxide 1 [15] M34. (i) same number of protons and electrons accept equal numbers of protons and electrons do not accept they are neutral 1 (ii) same element accept all atoms are potassium 1 same number of protons accept same atomic number accept they all have 19+ 1 different number of neutrons accept different mass numbers do not accept different atomic masses 1 [4] M35. (a) substance brokendown / separates / splits into elements by electric current / electricity ions free to move e.g. when molten / in solution allow 1 mark for “a substance that conducts electricity” max 2 (b) (i) copper / Cu 1 (ii) oxygen /O2 Page 124 allow CO2 1 (c) tube over electrode full of CuSO4(aq) / water allow sulphuric acid / sensible electrolyte not any other liquid / using a syringe 2 (d) Cu2+ ions removed / less Cu2+ not copper sulphate removed allow 1 mark for “copper removed / less copper” 2 [8] M36. (a) (i) H2O must be formula 1 (ii) catalyst 1 (b) (i) correct plotting 2 1 mark deducted per error to a maximum of 2 Page 125 do not accept a complete dot-to-dot line do not accept a bar chart if the (0,0) point is missing and line to one minute missing then maximum mark is 2 best fit single line if curve correct but no obvious points award 3marks 1 (ii) 4.5 – 5 no units required 1 (iii) all hydrogen peroxide had reacted accept all hydrogen peroxide had decomposed or been used up accept no hydrogen peroxide (particles) left 1 (c) (i) remains lower than previous line do not accept bar chart 1 line levels off lower than 60cm3 correct points but no line drawn then maximum 1 mark 1 (ii) decrease of (hydrogen peroxide) concentration accept concentration is less accept fewer collisions (of particles) do not accept weaker solutions or dilute solutions 1 [10] M37. (i) can be from diagram chlorine (2.8).7. accept chlorine needs one more electron 1 Page 126 can be from diagram shares a pair of electrons 1 shared pair of electrons is a covalent bond do not accept ionic bond 1 (ii) can be from diagram and appropriately annotated sodium (2.8). 1. and chlorine (2.8).7 1 sodium loses one electron and chlorine gains one electron 1 Na+ and Cl– formed 1 bond formed between oppositely charged ions or ionic bond is formed do not accept covalent bond 1 [7] M38. (i) a reaction in which the products can be changed back to reactants accept a reaction that can go forwards or backwards 1 under certain conditions 1 (ii) Mr CaCO3 = 100 1 Mr CaO = 56 1 mass of CaO = 140 (tonnes) 1 mark consequentially [5] Page 127 M39. (a) (i) test: limewater accept calcium hydroxide solution 1 result: ‘goes’ cloudy accept white or milky do not accept misty or chalky test must be correct before result mark can be considered 1 (ii) 2 NaHCO3 + H2SO4 → Na2SO4 + (2) H2O + (2) CO2 1 correctly balanced 1 (b) (i) H+ + OH– 1 → H2O deduct one mark if incorrectly balanced accept H3O+ instead of H+ then 2H2O needed for balance 1 (ii) pH increases accept numerical indication 1 (c) addition of sulphuric acid 1 correct use of an indicator accept idea of forming a neutral solution 1 crystallisation (of neutral solution) accept description using evaporation 1 [10] Page 128 M40. (a) breakdown / decomposition / splits into elements / not ions separates into elements / produce a chemical reaction 1 using electricity 1 (b) lead bromide melted / free ions not electrolyte 1 (c) (+) bromine element must be appropriate to electrode 1 (–) lead element must be appropriate to electrode 1 (d) fume cupboard / protective clothing allow safety glasses not safety mat 1 [6] M41. (a) exothermic (reaction) 1 (b) smaller lumps react faster or larger lumps react slower accept smaller lumps cause a more rapid rise in temperature Page 129 or vice versa do not accept higher temperature or more heat unless linked to time 1 smaller lumps have a larger surface (area) or larger lumps have a smaller surface (area) more water can react at the same time or so less water can react at the same time 1 (c) heats up (too) rapidly accept temperature (too) high 1 burning the food or the hands accept danger of container exploding or splitting or food overheating do not accept reference to handling of powder do not accept a lot of powder needed or powder getting into food or too hot to eat or food would not cook properly or heat through properly 1 [5] M42. (i) Mg + (H2SO4) → 1 MgSO4 + 1 H2 deduct 1 mark if not balanced only if all three correct accept alternative metal of similar reactivity for example Zn or Fe candidate would not then be awarded first mark for Mg then error carried forward deduct 1 mark if not balanced only if all three correct 1 (ii) to remove the (excess) magnesium accept separate accept insoluble substances or solids or residue Page 130 do not accept unreactive substances or impurities or remove magnesium from sulphuric acid 1 (iii) to evaporate (some of the water or solution) 1 to form crystals or crystallise accept to form a saturated solution or concentrated solution do not accept to leave MgSO4 1 [6] M43. (a) (i) Na2S2O3(aq) + 2 HCl(aq) → 2NaCl(aq) + H2O(l) + S(s) + SO2(g) 1 (ii) (formation of) sulphur accept precipitate or solid produced do not accept goes cloudy or milky 1 (b) (i) heat ≡ temperature increased temperature increases (the rate of reaction) or decreased temperature decreases rate of reaction may be gained in part (ii) if stated and not implied 1 (ii) (these ideas may be given in (i)) particles have more kinetic energy accept particles move faster 1 more collisions (so more reactions) more energetic collisions two marks 1 [5] Page 131 M44. (a) put on soil or for plants accept land or field or garden or crops or plants accept alternative answer to provide more food for increased population for growth accept to improve plant yield or help them grow accept to replace or add nutrients (not nitrates) or minerals or to make plants grow better or for healthy plants do not accept to make soil fertile or to feed plants 2 (b) (i) 2 1 (ii) 80 1 [4] M45. (a) calcium atom loses two electrons accept diagrams with correct labelling 1 (each) fluorine atom gains one electron accept two electrons transfer from a calcium atom to the two fluorine atoms for these first two marks 1 forming full (outer) shells of electrons accept forming full (outer) energy levels or noble gas electronic structures do not accept stable unless qualified 1 giving the ions Ca2+ and F 1 Page 132 attraction between ions of opposite charges accept electrostatic attraction between ions if candidate mentions sharing or pairing of electrons then no credit if explanation is entirely correct but they state this is called covalent bonding, the maximum mark is four 1 (b) atoms of the same element 1 atomic number is same accept each contains 92 or same number of protons 1 mass numbers differ or each has a different number of neutrons 1 one has 146 neutrons the other has 143 neutrons accept one has three more or less neutrons than the other 1 (c) (i) 349 1 (ii) 349g UF2 produces 235g U [1] first mark can be awarded if answer is incorrect answer = 117.5 1 [12] M46. (i) electrolysis 1 (ii) oxidation 1 (iii) hydroxide ions or OH– accept sodium hydroxide or hydroxide or OH for one mark only Page 133 2 (iv) H+ + e– 1 H2 ignore any state symbols 1 2H+ + 2e– → H2 accept H+ + e-→ H for one mark only 1 [7] M47. (a) (i) bulb lights up 1 bubbles / fizz / gas or chlorine given off 1 (ii) in solid, ions 1 are not free to move / (charged) particles cannot move or converse atoms / electrons cannot move worth 0 marks 1 (b) (i) breakdown / decomposition / splitting up not separation 1 by using electricity 1 (ii) gas A = chlorine / oxygen 1 deposit B = copper 1 Page 134 (c) any one from: • manufacturer of chlorine / sodium hydroxide / hydrogen / sodium • electroplating of steel / reference to plating not galvanising • extraction of aluminium / metal reactivity series specified • purification of copper not making copper 1 [9] M48. (a) (i) H2O2 reactant correct ignore any state symbols 1 H2O + O2 products correct 1 2H2O2 → 2H2O + O2 balanced accept correct multiple 1 (ii) glowing splint 1 relights accept ‘bursts into flame’ do not accept a lighted splint burns brighter or faster 1 (b) unchanged accept not used up or left (behind) 1 (c) (i) gas syringe or measuring cylinder either with scale drawn or labelled 1 the apparatus as drawn would work 1 Page 135 (ii) correct plotting of points one mark to be deducted for each error 2 best fit graph line drawn (single line drawn) 1 (iii) concentration of hydrogen peroxide decreases accept less particles of hydrogen peroxide to collide do not accept hydrogen peroxide gets used up 1 rate of reaction decreases accept reaction gets slower 1 (iv) any two from: • temperature • pressure • division of catalyst or manganese oxide do not accept any other factors 2 [15] M49. 160 ignore units if answer incorrect then (2 × 56) + (3 × 16) or 112 + 48 for one mark [2] M50. (a) (i) Page 136 calcium carbonate → calcium oxide + carbon dioxide accept CaO3 → CaO + CO2 1 (ii) (thermal) decomposition accept endothermic accept reversible 1 (b) (i) neutralisation accept exothermic 1 (ii) sulphuric (acid) H2SO4 2 (c) (i) to speed up the reaction accept to increase the rate of reaction or to increase the number or rate of collisions do not accept “dissolves” copper oxide faster 1 (ii) all acid reacts accept there will be no acid left or acid used up 1 acid is neutralised (for 2 marks) do not accept to form a concentrated or saturated solution 1 (excess) copper oxide collects in filter paper accept larger particles (of copper oxide) cannot pass through filter paper 1 copper sulphate solution passes through the filter paper accept dissolved copper sulphate passes through filter paper or smaller particles (of copper sulphate) in solution (liquid) pass through filter paper accept (black) solid collects in filter paper and filtrate or Page 137 soluble solid or (blue) solution (liquid) passes through filter paper for 1 mark only 1 [10] M51. (i) convection currents accept a suitable description of convection currents 1 move the Earth’s plates accept a suitable description of ‘movement’ of Earth’s plates 1 at plate boundary one plate or a slab of rock can be pushed down forming magma/molten rock accept at subduction zones magma/molten rock is formed – deconstructive boundary 1 magma/molten rock rising and cooling at the Earth’s surface reforms as part of the plate accept magma/molten rock rising and cooling at the Earth’s surface forms igneous rock – constructive boundary accept Earth’s crust or lithosphere for Earth’s surface 1 (ii) isotopes are atoms of the same element do not accept that isotopes have the same atomic number but a different atomic mass 1 19/the same number of protons 1 19/the same number of electrons do not penalise for incorrect 1 20 and 21 neutrons/different numbers of neutrons arithmetic if concept is correct 1 [8] Page 138 M52. answers apply to: accept diagrams and/or descriptions carbon dioxide CO2 ammonia NH3 methane CH4 water H2O *outer electronic structure of one atom correct or needs correct number of electrons to complete outer shell 1 *outer electronic structure of other atom correct or needs correct number of electrons to complete outer shell 1 *one shared pair of electrons (as one covalent bond) use of ions or reference to ionic bonding negates this mark 1 *outer electronic structure of compound correct or each atom now has a full outer shell/noble gas electron structure 1 [4] M53. (a) (i) must be chemical symbol Ca 1 C CaCO3= 2 marks 1 O not O2 1 Page 139 (ii) carbon dioxide must be name 1 (b) (i) points all correct 2 marks one point incorrect 1 mark two points incorrect 0 marks 2 suitable line -narrow neat single curve not dot to dot 1 (ii) reaction with X forms less gas must include X or Y do not penalise for H2/O2 if (a) (ii) already penalised do not accept is finished in less time or slower/faster reaction or lower on graph 1 (iii) any two from: • concentration (of acid) decreases/less reacting particles/molecules not acid/CaCO3 runs out/is used up • surface area of calcium carbonate decreases not strength of acid decreases • less collisions between reacting particles not smaller (amount of) CaCO3 2 [10] M54. (a) (i) to remove or separate copper oxide accept to remove or separate unreacted or excess base accept to remove or separate insoluble solids 1 Page 140 (ii) heat (the solution) accept heat the water accept evaporate the water rapid cooling/cool to lower temperature accept boil the water or solution not increase surface area, put in draught not increase the temperature 1 (iii) aqueous accept in water accept solution not soluble in water 1 (b) add water/liquid/solution 1 colour changes to blue 1 [5] M55. 352 g gains 3 marks (moles C8H18 = 114 / 114 = 1 mole) moles CO2 = 8 (1) mass CO2 = 8 × 44 (1) = 352 g (1) 1 mark for each point (ecf allowed between parts) or 114 → 8 (1) × 44 (1) 114 → 352 g (1) ecf allowed between parts [3] Page 141 M56. hydrogen ions (from acid) or protons / H+ 1 react with hydroxide ions (from alkali) / OH 1 to produce water H + OH H2O gains all 3 marks ignore state symbols molecules of hydrogen ions and molecules of hydroxide ions produce water = 2 marks if they fail to get any of the above marks they can get 1 mark for neutralisation / product neutral 1 [3] M57. (i) to make sure all sulphuric acid reacts or to neutralise the acid or unreacted sulphuric acid difficult to remove owtte ignore ‘to maximise the product’ accept otherwise (sulphuric) acid left 1 (ii) filter(ing) / filtration or described owtte accept use filter paper accept centrifuge and decant do not accept sieve / strain filter funnel is insufficient 1 (iii) no more solid / solute can dissolve or maximum amount of solid owtte 1 at that temperature accept any link to temperature or any specified temperature 1 Page 142 (iv) solubility decreases (as temperature falls) owtte accept less soluble in cold water answer must be linked to solubility ignore the extra cannot dissolve 1 (v) otherwise get anhydrous CuSO4 accept otherwise get white CuSO4 accept do not get hydrated CuSO4 accept could get CuO or thermal decomposition / decomposes allow SO3 / SO2 produced allow dehydration accept removes the water of crystallisation not just remove water from the crystals or just steam 1 [6] M58. (a) all electrons correct (inner shell need not be shown) three bond pairs and two electrons anywhere else can use dots, crosses or e’s in any combination 1 (b) covalent accept phonetic spelling do not accept convalent 1 [2] M59. (a) 6 accept 5.8 – 6 1 (b) hydrochloric acid used up / reacted / combined / or fewer particles Page 143 (of hydrochloric acid) or fewer hydrogen ions owtte accept reactants used up accept less calcium carbonate or smaller surface area of calcium carbonate accept lower concentration / less crowded do not accept atoms / molecules ignore references to energy do not accept references to atoms or molecules 1 fewer collisions owtte independent mark 1 (c) steeper curve initially independent marks 1 levels out at same volume • must indicate levelling out • if line goes higher than 66 do not award this mark • diagonal line only = 0 marks • if steeper initially and then crosses the line and finishes correctly, then loses one 1 [5] M60. (a) A ammonia accept correctly indicated in the box 1 B nitrogen monoxide 1 C nitrogen dioxide 1 (b) any two from: • air / oxygen / O Page 144 • ammonia / NH • water / H O • nitrogen / N 2 (c) (i) speeds up reaction (owtte) 1 (ii) platinum accept indication, circles etc 1 [7] M61. (a) sodium hydrogen phosphorus oxygen 2 marks for all 4 1 mark for 2 or 3 0 marks for 0 or 1 not symbols / formulae 2 (b) (i) gives out gets hot(ter) / temperature rises (1) 1 heat / energy independent mark 1 (ii) Quality of written communication for clearly expressed ideas 1 take temperature of water at start owtte 1 take temperature after adding soup powder 1 Page 145 plus any one from: • using a thermometer • mix / stir / shake etc • in beaker / conical flask / test tube / plastic cup • temperature will rise (indicates an exothermic reaction) 1 [8] M62. (a) (i) water accept H2O accept correct ringed answer in box 1 (ii) neutralisation accept underlining or any indication, eg tick 1 (b) sodium hydroxide 1 sulphuric acid apply list principletotal 1 [4] M63. (a) Mr (SiO2) = 60 if Mr incorrect ecf for max 2 1 60 g SiO2 → 28 g Si correct answer for 3 marks 1 2.14 g SiO2 → 1 g Si allow 2, 2.1, 2.14 (or anything rounding to 2.14), 2.16 or 2.2 a unit is not required but an incorrect unit loses the third mark Page 146 OR Mr (SiO2) = 60 (1) moles if silicon needed = = 0.0357 mass of SiO2 needed = 0.0357 × 60 (1) = 2.14 g (1) allow 2, 2.1, 2.14 (or anything rounding to 2.14), 2.16 or 2.2 OR Mr (SiO2) = 60 (1) mass SiO2 = 1 × (1) = 2.14 g (1) allow 2, 2.1, 2.4 (or anything rounding to 2.14), 2.16 or 2.2 3 (b) (i) MgO(s) + 2HCl(aq) → MgCl2(aq) + H2O(l) penalise incorrect symbols correctly balanced equation for 1 mark state symbols for 1 mark allow correct multiples / fractions 2 (ii) or ignore inner shell electrons of silicon allow correct drawings without symbols must clearly indicate four shared pairs of electrons with one electron from each atom Page 147 (iii) Si H 1 = 0.05 = 0.15 1 1 3 for whole number ratio can be implied 1 Si H3 accept H3 Si or any correct formula with 1:3 ratio if in step 1 they get either of ratios incorrect they lose first 2 marks but can be ecf for 3rd and 4th mark evidence of mass / Ar 1 mark proportions of each 1 mark whole number ratio 1 mark correct formula 1 mark 1 (iv) C accept c 1 (c) any four from: • giant structure / macromolecule / lattice / giant molecule allow giant molecular / giant atomic structure • each silicon atom joined to four other atoms (or diagram) • covalent bonds • bonds are strong or large amount of energy needed to break bonds accept hard to break bonds • large number of bonds to be broken mention of giant ionic structure or intermolecular forces or intermolecular bonds max 1 mark diamond or carbon discussion max 3 marks unless clearly linked to silicon 4 [15] Page 148 M64. (a) all lead nitrate reacted or no lead nitrate left or enough KI to react with lead nitrate or to remove all the lead ions or to get maximum amount of I2 ignore comments about speed do not accept to remove all the lead 1 (b) precipitate allow phonetic spelling do not accept ppt 1 1 (c) filter / filtration / centrifuge / decant do not accept sieve (d) any one from: • removes (soluble) impurities • removes (unreacted) KI • removes KNO3 • removes (excess) solution • removes nitrates purifying is insufficient do not accept removes potassium do not accept removes iodide 1 (e) answer based on filter paper, desiccator, suitable solvent (gentle) heat, drying cabinet, oven etc. Accept any method of heating i.e. bunsen / hairdryer etc. Accept leave to evaporate / stand or leave in a warm room e.g. place between dry filter paper, allow to dry e.g. use propanone, allow to dry e.g. leave on sunny window sill e.g. leave in a draught the answer leave / evaporate / draught alone is insufficient 1 Page 149 (f) wear gloves / mask or fume-cupboard or wash hands afterwards ignore goggles / labcoat or extractor fan / do not touch etc. 1 [6] M66. (a) 100 ignore units 40 + 12 + (3 × 16) for 1 mark 1 (b) 40 (ecf from part (a) can get 2 marks) for 1 mark 1 (c) 0.5 (ecf from part (b) can get 2 marks) or other correct working for 1 mark 2 (d) gas produced or carbon dioxide / CO2 produced 1 [7] M67. (a) hydrogen accept H2 do not accept H 1 Page 150 (b) litmus paper / Universal Indicator paper / pH paper allow any suitable named indicator 1 bleached / turns white or loses its colour do not accept bleached cloth / leaves etc. allow second mark unless incorrect indicator given allow starch iodide paper (1) goes black / blue black (1) allow potassium iodide solution (1) goes brown / orange / black precipitate (1) 1 (c) because they have a negative charge or opposite charges attract accept (because) it is Cl– accept chlorine, Cl or chlorine ions has a negative charge do not accept Cl– on its own do not accept Cl2 o.e. has negative charge 1 (d) kill bacteria / germs, etc. or sterilise / disinfect accept destroys bacteria etc. ignore clean / purify water (owtte) do not accept just gets rid of bacteria 1 (e) hydroxide (ion) accept OH– 1 [6] M68. (a) (i) high and low both needed for mark 1 (ii) reversible 1 (iii) to prevent ammonium chloride / solid / particles escaping Page 151 idea of a filter do not accept ‘to prevent gases escaping’ 1 (b) endothermic 1 [4] M69. nitric acid 1 potassium hydroxide 1 water 1 [3] M70. (i) measure volume / mass of gas produced 1 in a certain time period 1 mark is for a sensible way of measuring the amount of product produced and 1 mark is for the idea of timing e.g. measure volume of gas produced at regular time intervals or time taken to fill a test tube with the gas or collect a certain volume of gas (measuring the rate at which bubbles are produced e.g. number of bubbles in 30 seconds gains only 1 mark unless an enclosed system is used) or measure decrease in mass of flask and contents at regular time intervals or time taken for the mass to decrease by certain amount 1 (ii) increases rate (owtte) 1 (ii) change the concentration or add a catalyst or change the surface area or lower the temperature Page 152 accept ‘expose to sunlight’ (owtte) or change the amount of water / powder / solution used ignore ‘stirring’ 1 [4] 168g → 44g M71. 1 1g → 1 11g → 2.88g (2.9g) care with rounding 1 or Mr values 84 and 44 (1) moles hydrogen carb = (1) mass of CO2 = = 2.9g answer 2.88 to 2.9 gets 3 marks answer of 3 gets 2 marks (1) [3] Page 153