Supplementary Information (doc 3546K)
advertisement

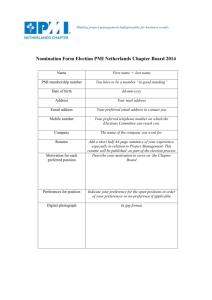
Table S1. Data collection and refinement statistic Data Collection Space group Cell parameters, Å Complex/a.u. Resolution a, Å Number of reflections Total Unique Rmergeb, Completeness, % Redundancy I/σ, (I) Refinement Statistics Resolution, Å Rc, % Rfreed, % No. of protein atoms No. of ligand atoms No. of water molecules Root mean square deviation Bond lengths, Å Bond angles, ° Ramachandran plot Most favored region, % Additional allowed region, % Generously allowed region, % Disallowed region, % P1 a=68.98, b=213.48, c=45.54 2 50-1.53 (1.58-1.55) 52,590 27,679 5.9 (48.5) 93.0 (87.4) 1.9 (1.7) 16.1 (1.1) 38.67-1.55 18.3 22.3 1324 190 141 0.012 1.44 92.8 7.2 0.0 0.0 a all data (outer shell). Rmerge = ∑│I - <I>│/∑I, where I is the observed intensity and <I> is the average intensity obtained from multiple observations of symmetry-related reflections after rejections c R = ∑║Fo│- │ Fc║/∑│Fo │, where Fo and Fc are the observed and calculated structure factors, respectively d Rfree = defined by by Brünger b 1 Figure S1. Native chemical ligation of (24-75)MDMX-αCOSCH2CH2COLeu to (76-108)MDMX (left panel – A,B,C) and pTyr99-(76-108)MDMX (right panel – D,E,F). The reactions were carried out in 0.1 M phosphate buffer containing 6 M guanidine hydrochloride (GuHCl) and 50 mM MPAA, 20 mM TCEP, pH 7.4, and monitored at different time intervals. Upon completion, the ligation products, (24108)MDMX and Tyr99-(24-108)MDMX, were purified to homogeneity by preparative C18 RP-HPLC. Protein folding was achieved by dissolving the polypeptide in 6 M GuHCl at 1 mg/mL, followed by a 6fold dilution with PBS, pH 7.4, and an extensive dialysis against the same buffer. Protein quantification was done spectroscopically by UV measurements at 280 nm using a molar extinction coefficient of 7450, calculated according to the published algorithm developed by Pace and colleagues(1). 2 Figure S2. Purified (24-108)MDMX (A) and its pTyr99 analogue (B) characterized by C18 RPHPLC and ESI-MS. The chromatograms were obtained at 40 C on a Waters XBridge C18 column (4.6 × 150 mm, 3.5 m) running a 30-min gradient of 5-65% acetonitrile in water containing 0.1% TFA at a flow rate of 1 ml/min. The observed masses of 9735.9 ± 0.5 Da and 9815.0 ± 0.6 Da were within experimental error of the theoretical values of 9735.5 Da and 9815.5 Da calculated for MDMX and pTyr99 MDMX, respectively, on the basis of their average isotopic compositions. 3 Figure S3. Circular dichroism (CD) spectra of (24-108)MDMX and pTyr99-(24-108)MDMX in the absence (A) and presence (B,C) of (15-29)p53. The spectra were collected at room temperature at 10 M in 10 mM phosphate buffer, pH 7.2. Addition of an equal molar concentration of the p53 peptide to the MDMX proteins enhanced alpha helicity due to the formation of a helical ligand conformation in the complex. However, the enhancement for pTyr99 MDMX was significantly smaller than for MDMX, suggesting that the binding affinity of p53 for the phosphorylated protein was lower than that for the wild type protein. 4 Figure S4. GuHCl-induced two-state denaturation of MDMX and pTyr99 MDMX monitored at room temperature by CD spectroscopy at 222 nm. The experimental details were as described previously(2, 3). Briefly, an increasing amount of aliquot was withdrawn from an initial 2.5 mL of native protein, 10 M in 10 mM phosphate buffer, pH 7.2, prepared in a 1-cm cuvette, followed immediately by addition of an equal volume of denatured protein of the same concentration, prepared in 10 mM phosphate buffer containing 7.2 M GuHCl (pH 7.2). This procedure generated a stepwise increase (0.25 M) in the concentration of GuHCl in the cuvette from 0 to 5 M after 20 withdrawal/addition cycles. The solution in the cuvette was thoroughly mixed before signals at 222 nm were recorded at different GuHCl concentrations. The experimental data were subjected to a six-parameter nonlinear regression analysis by using a published equation(2, 3), yielding the free energy change of unfolding, ΔΔG (kcal/mol), at zero GuHCl concentration. The ΔΔG values were 2.5 ± 0.2 kcal/mol for MDMX and 2.8 ± 0.3 kcal/mol for pTyr99 MDMX. 5 Figure S5. Superimposition of the two copies of PMI-pTyr99 MDMX complexes present in asymmetric unit of crystal. Cα- traces of the two copies of PMI-pTyr99 MDMX complexes are shown as ribbons, whereas the modified tyrosine at position 99 of MDMX and the residues on the PMI peptide are shown as ball and sticks. The experimental electron density clearly defined residues 2-11 and 2-10 of PMI on first and second copy of the complex in the asymmetric unit. Overall structures of these copies are very similar with the root mean square deviation (RMSD) of equivalent Cα atoms of 0.32 Ǻ. Conformation of PMI molecules also remain very similar as reflected by RMSD of 0.02 Ǻ of 10 backbone atoms. 6 Figure S6. Superimposition of the p53-MDMX (blue/grey) and PMI-MDMX (cyan/red) complexes. Structures were aligned based on MDMX molecule from PMI-MDMX complex (PDB code 3EQY(4) and are shown as ribbon representation. Phe3 & 19, Trp7 & 23 and Leu10 & 26, Ser11, Pro27, Asn29 of PMI/p53 and Tyr99 of both the complexes are shown as sticks. 7 Figure S7. Superimposition of the PMI-pTyr99 MDMX (yellow/green) and p53-MDMX (magenta/blue) complexes. (A) Structures were aligned based on MDMX molecule from PMI-MDMX complex (PDB code 3EQY(4)) and are shown as ribbon representation. Phe3 & 19, Trp7 & 23 and Leu10 & 26, Ser11, Pro27, Asn29 and Tyr99 of both the complexes are shown as sticks. (B) Close up views into overlaid PMI-pTyr99 MDMX and p53-MDMX complex interfaces. Residues of PMI-pTyr99 MDMX /p53MDMX which contribute to the binding are shown as sticks. 8 REFERENCES 1. Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4(11):2411-23. 2. Lu WY, Starovasnik MA, Dwyer JJ, Kossiakoff AA, Kent SB, Lu W. Deciphering the role of the electrostatic interactions involving Gly70 in eglin C by total chemical protein synthesis. Biochemistry. 2000;39(13):3575-84. 3. Zhan C, Varney K, Yuan W, Zhao L, Lu W. Interrogation of MDM2 phosphorylation in p53 activation using native chemical ligation: the functional role of Ser17 phosphorylation in MDM2 reexamined. Journal of the American Chemical Society. 2012;134(15):6855-64. 4. Pazgier M, Liu M, Zou G, Yuan W, Li C, Li C, et al. Structural basis for high-affinity peptide inhibition of p53 interactions with MDM2 and MDMX. Proc Natl Acad Sci U S A. 2009;106(12):466570. 9