The role of the Renal System in acid
advertisement

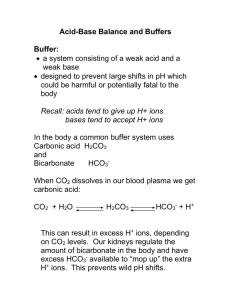
1 The role of the Renal System in acid-base balance Reading: Egan’s pp. 277 – 281, 290 – 294 There are two organs that primarily excrete acid produced by waste products from metabolism I. II. Lungs – The Lungs can remove CO2 in large quantities at a rapid pace. Kidneys – The kidneys also have the ability to remove CO2, but at a slower pace. In healthy individuals, there is a balance between the two organs to maintain homeostasis. When a person is sick, failure in one organ can be offset by the compensatory actions of the other. LUNGS The Lungs can only excrete the CO2 from disassociating H2CO3: CO2 + H2O H2CO3 HCO3- + H+ The Lungs do not physically remove H+ ions; rather it eliminates the CO2 from the blood during exhalation and attaches the H+ ion to create the H2O molecule. KIDNEYS I. Key terminology for the renal system: a. Excretion – the elimination of a substance in the form of urine b. Secretion – process where renal tubules cells actively transport substances into the tubule lumen’s fluid c. Filtrate – the tubule lumen’s fluid d. Reabsorption – active or passive transport of filtrate substances back into the tubule cell and then into the bloodstream II. The renal system functions in two ways to balance the acid: Excrete H+ ions Retain HCO3- 2 III. The Nephron a. The functional unit of the kidney b. It is composed of a series of blood vessel surrounding a renal tubular c. There are over a million nephrons in the kidneys d. Its function is to filter the blood, reabsorb essential substances, and excrete nonessential molecules and waste e. Due to hydrostatic pressure, water leaves the capillaries to enter the renal cells that surround the filtrate that will eventually become urine. The water passes the renal cells to enter the filtrate. f. As the blood volume increases to the renal nephron, there is an increased volume of water leaving the capillary to enter the filtrate. g. Substances in this water such as CO2, HCO3- and H+ ions also leave the capillaries to enter the renal cells and pass through into the filtrate h. Inside the tubular lumen, as the filtrate moves along, the H+ ions are captured by various substances (buffers) which stop them from reentering the blood stream. i. As the H+ ions leave, the pH of the blood rises. j. Simultaneously 80-90% of HCO3- is being reabsorbed into the bloodstream to raise the pH of the blood There are several mechanisms that contribute to the renal system’s role in acid-base balance. I. As CO2 enters the renal cell, HCO3- and H+ are created by the rapid CO2 hydrolysis with the presence of Carbonic Anhydrase in the renal system. The result of this chemical reaction is a large amount of HCO3- that needs to return to the bloodstream and a large amount of H+ ions that needs to stay in the filtrate. II. The renal cells actively transport H+ ions into the filtrate via countertransport in which Na+ (sodium) exchanges places with H+ ions. When the Na+ enters the capillary, it combines with HCO3- to become NaHCO3, which cannot diffuse back into the filtrate. III. Simultaneously, HCO3- does not easily diffuse out of the renal cell. It can return to the bloodstream as CO2 due to the rapid hydrolysis of CO2 inside the renal cell. a. Because there is so much H+ ions and HCO3- the equation moves back into CO2 and H2O. Now, CO2 can easily re-enter the bloodstream. b. As The CO2 enters the bloodstream, it again may undergo hydrolysis and the resulting HCO3- is in the bloodstream. 3 c. The Na+ was exchanged with the H+ in the filtrate will now capture the CHO#- to become NaHCO3. The HCO3- will now remain in the bloodstream. IV. Ammonia buffer (NH3) a. The catabolic metabolism of amino acids into human protein produces a large amount of a strong base called ammonia. In the liver, this poisonous gas is converted into urea and water. b. Once in the filtrate, ammonia can accept an H+ ion, resulting in NH4+ (ammonium ion). The ammonia is now a buffer because it captured the H+ ion. c. When there is a large amount of H+ ions in the blood, thus it is also increased in the filtrate; the renal tubular cells can produce more ammonia to capture more H+ ions. d. While the ammonia (NH3) accepts the H+ ion, a Cl- ion is secreted by the kidney to maintain electroneutrality. e. This action frees up a Na+ ion to combine with the HCO3- so that the HCO3- can remain in the bloodstream. V. Phosphate buffer – Phosphate (HPO4-) accepts an H+ ion and becomes H2PO4-, therefore more acid is excreted in the urine. Renal failure and the Cardiopulmonary System The healthy human renal system creates and excretes 1-2 L/day of urine. The urine of the healthy person will have an average pH of 6.0, which can vary with diet. Signs of renal failure include: I. Metabolic acidosis a. Increased metabolic acidosis is naturally corrected by and increased respiratory effort. b. The lungs can only bring the PaCO2 down to 12 torr under the best circumstances; therefore there is only partial compensation of metabolic acidosis. II. Creatine – derangements of several serum chemicals such as creatine can result from renal failure. Presence of this protein in the urine is a serious sign of damage to the capillaries in the renal system. 4 III. Urine output a. A decrease in the volume of urine put out by the patient is another sign of renal failure. b. When there are serious problems with the kidneys, the patient can retain fluid, which places excess water in the blood stream. c. This action increases blood volume, creating systemic hypertension, placing stress on the heart. When the blood volume increases beyond the ability of the left ventricle to send the blood out of the heart, blood backs up and floods the pulmonary capillary bed. Due to J receptors, the patient will start to breathe rapidly and shallowly. The resulting excessive hydrostatic pressure will flood the pulmonary interstitial space. The alveoli will require increased pressure to inflate. The patient’s compliance will go down and the WOB will increase more. Due to Fick's law of gas diffusion this thickened membrane will decrease the diffusion of O2 into the capillaries, which could result in refractory hypoxemia. Renal failure can lead to decreased diffusion of O2 as well as decreased compliance, as the engorged lung becomes stiff and non-compliant. There are limitations to respiratory compensation of metabolic problems: The renal system can compensate for respiratory acid/base balances completely. The lung is less able to fully compensate for metabolic problems because: The body can only raise CO2 to 50 mmHg to compensate for metabolic alkalosis - because you have to breathe due to your hypoxic drive (peripheral chemoreceptors) The body can only drop CO2 to the teens because the Ve is limited by finite surface area and muscle fatigue. PO2 can only drop to 50mmHg then your peripheral chemoreceptors note the aortic hypoxemia and you must breathe When PAO2 drops low enough to cause vasoconstriction of the pulmonary bed, the J receptors trigger rapid, shallow breathing. Which means… The body is too “smart” to stop breathing to compensate for a renal alkalosis.