476 - South West Yorkshire Partnership NHS Foundation Trust
advertisement

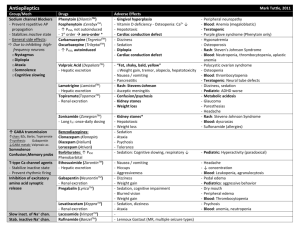
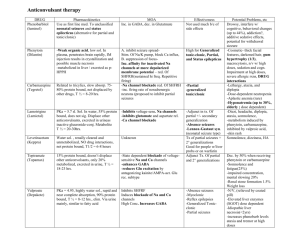
Portfolio Medicines Management Document name: Carbamazepine Communication Document type: Pharmacy Communication Staff group to whom it applies: Prescribers All qualified nursing staff All pharmacy staff Distribution: Trustwide How to access: Intranet PA to Chief Pharmacist Issue date: March 2006 Reviewed: December 2009 January 2012 January 2014 Next review: Approved by: Drug and Therapeutics Trust Action Group Developed by: Director leads: Kate Dewhirst, Deputy Chief Pharmacist Katie Crowe, Senior Clinical Pharmacist Dr Booya, Medical Director Contact for advice: Med.information@swyt.nhs.uk Focus on Carbamazepine in Mental Health Introduction Carbamazepine is licensed for the prophylaxis of bipolar disorder in patients unresponsive to lithium therapy. Evidence of efficacy is uncertain. It may be beneficial in rapid cycling bipolar disorder. Additionally it is licensed for generalised tonic-clonic and partial seizures and the paroxysmal pain of trigeminal neuralgia. It is also used for aggression in schizophrenia, attention-deficit hyperactivity disorder, and self-injurious behaviour, use for these indications will require completion on an unlicensed use form as outlined in Section 17 of the Medicines Code. . Dosage and administration The modified release form of Carbamazepine is recommended. This is prescribed orally, with the total daily dose given as either a single dose at night or as two divided doses. It can be given, before, after or during meals. Liquid and chewable tablets are available if there are swallowing difficulties. For the prophylaxis of bipolar disorder in patients (unresponsive to lithium) the recommended starting dose of carbamazepine is 400mg daily in divided doses, increasing gradually until symptoms are controlled or a dose of 1600mg is reached. Monotherapy is desirable in view of the high potential for both pharmacokinetic and pharmacodynamic interactions. Plasma level monitoring is established in the treatment of epilepsy, the therapeutic range being stated as 4- 12 mg/l. The evidence of a therapeutic range is less strong for affective illness. A serum level of at least 7mg/l has been suggested. This should be interpreted in conjunction with symptom assessments. For the purposes of consent to treatment carbamazepine can be authorised as: 4.2.3 Antimanic medication 4.8.1 Antiepileptic medications Carbamazepine The BNF maximum dose is 1600mg/day, so dosing must be within this where the BNF maximum is stated as part of the consent to treatment form. Contra indications Carbamazepine is contra-indicated in atrio-ventricular block, a history of bone marrow suppression or a history of acute porphyria. Due to its structural relationship to tricyclic anti-depressants, carbamazepine is not recommended in combination with monoamine oxidase inhibitors (MAOIs).Carbamazepine should never be co-prescribed with clozapine due to the potential to cause leucopenia. Adverse events and special precautions 1. Agranulocytosis and aplastic anaemia occur with a very low incidence. A decreased white cell count and platelet count is reported occasionally to frequently. Action - withdraw immediately if leucopenia is severe, is becoming progressively worse or there are clinical symptoms (sore throat, fever, bruising, mouth ulcers malaise, non-specific illness) Closer monitoring is required if there are low levels of abnormal results. 2. Severe hepatic reactions to carbamazepine occur very rarely. Abnormal results of liver function tests (LFTs) are not necessarily an indication of liver impairment. Levels of gamma-glutamyltransferase (GGT) and alkaline phosphatase (AP) may be raised due to enhanced hepatic metabolising capacity i.e. enzyme induction. Action - withdraw immediately in cases of aggravated liver dysfunction or acute liver disease, if LFTs are severely abnormal, are becoming progressively worse or there are clinical symptoms (jaundice, bruising, oedema, ascites hypoglycaemia, infection) Closer monitoring is required if there are low levels of abnormal results. 3. Severe skin reactions may occur e.g. Stevens-Johnson syndrome and Lyell’s syndrome (toxic epidermal necrolysis) Action - withdraw immediately is signs and symptoms suggest severe reaction. Mild skin reactions are mostly transient and disappear within a few days or weeks. Close surveillance is required for a worsening rash or accompanying symptoms (systemic illness, fever, arthralgia or myalgia, vesicles are present in the mucosa of the mouth, GI tract or conjunctivae. Rarely a delayed multi-organ hypersensitivity disorder may occur which can affect the skin, liver and other organs as above, alone or in combination. Treatment should be withdrawn immediately. Other very rare but potentially serious adverse events include renal failure, interstitial nephritis, neuroleptic malignant syndrome, hyponatraemia and serotonin syndrome. The most common problems associated with carbamazepine are dizziness, drowsiness, ataxia and nausea. These can be minimised by starting with a low dose and increasing gradually, or by using the slow-release formulation. Pregnancy Carbamazepine is estimated to increase the risk of neural tube defects from 6 in 10,000 to around 20 to 50 in 10,000 and carries a risk of other major fetal malformations including gastrointestinal tract problems and cardiac abnormalities. If a woman who is taking carbamazepine for mental health conditions is planning a pregnancy healthcare professional should advise her to stop taking the medication and if appropriate an alternative medication may be considered. If a woman is taking carbamazepine at the time of conception and/or in the first trimester of pregnancy health professionals should offer screening and counselling about the continuation of the pregnancy, the need for additional monitoring and the risks to the fetus if the woman continues to take the medication. The newborn will require a full paediatric assessment and subsequent monitoring. Clinically relevant interactions Antipsychotics Clozapine and carbamazepine must not be used together due to the additional risks of agranulocytosis. With all antipsychotics, CNS side effects may be additive e.g. drowsiness, ataxia. Risperidone may increase carbamazepine levels and carbamazepine may reduce risperidone levels. MAOIs Need two week washout period Lithium Neurotoxicity and increased risk of side effects of both drugs without raised serum levels Anticonvulsants Complex interactions with phenytoin, lamotrigine, sodium valproate and others. Monitoring of both medications will be required. Consult BNF, pharmacist or neurologist. Erythromycin Can rapidly increase carbamazepine levels by 100-200%. Use alternative or monitor carbamazepine levels. Grapefruit juice A single serving can increase carbamazepine levels. Oral contraceptives Leads to a reduced contraceptive effect. Use at least 50 micrograms of ethinylestradiol a day or Depo Provera every 10 weeks or other methods of contraception. Warfarin Metabolism of warfarin is accelerated and efficacy may be reduced. Doses may need to be increased by 100% to achieve target INR. Care required when carbamazepine is withdrawn, as INR will be increased with risk of bleed. Calcium channel blockers Diltiazem and verapamil may increase the risk of carbamazepine toxicity. Tricyclic antidepressants Plasma levels of the antidepressant are reduced Caution and increased frequency of monitoring is required when carbamazepine is used concomitantly with other medication that may cause similar adverse events. Recommendations Baseline and ongoing monitoring is required. Full blood count Baseline then 2 weekly for 2 months then 3-6 monthly Hepatic function Baseline and 3-6 monthly As most hepatic adverse event occur in the first month, consider earlier monitoring if signs and symptoms of hepatic dysfunction are present Serum carbamazepine To ensure therapeutic level is reached 2-4 weeks after dose changes then 3-6 monthly Renal function and Baseline and 6 monthly electrolytes (Complete urinalysis and BUN are recommended by the SPC) Carbamazepine should only be prescribed after a benefit–risk appraisal and under close monitoring in patients with a history of cardiac, hepatic or renal damage, or adverse haematological reactions to other medication. Carbamazepine is not recommended for acute mania in part due to the time taken to reach therapeutic levels. Ensure service user and or carer is aware of potential serious adverse events and the signs and symptoms to be mindful of. Provide verbal and written information. Continually review efficacy and symptom control, particularly in unlicensed indications to ensure on-going benefit outweighs the risks. Document this review All results, including normal results and any actions required should be recorded in the medical notes LFT results should be considered as a combination rather than single results. Generally, alanine aminotransferase (ALT) may be considered a specific marker for acute hepatocellular damage and albumin, bilirubin and prothrombin time indicate how well the liver is functioning. A mildly elevated ALT value (less than 1.5 times normal) could be normal for gender ethnicity or body mass index. LFTs can be normal in those with chronic hepatitis and cirrhosis. Due to the difficulty in interpreting results, clinical monitoring is of primary importance throughout treatment References Summary of Product Characteristics; Tegretol; Novartis Pharmaceutical UK Ltd Wyeth; February 2011 The Maudsley Prescribing Guidelines 10th Edition 2009. South London and Maudsley NHS Trust, Oxleas NHS Trust Psychotropic Drug Directory, 2010; Bazire BNF www.bnf.org.uk Mason P. Blood tests used to investigate liver, thyroid or kidney function and disease. PharmJ 2004; 272; 446-448 Johnston DE. Special considerations in Interpreting Liver Function Tests. American Family Physician 1999; 59; Nickless G. An overview of hepatitis. Pharm J 2008:280:411-414 NICE CG 137: The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care. Jan 2011.