Practice -3
advertisement

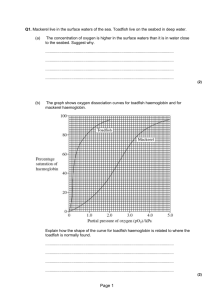
College of Health Sciences Department of Medical Laboratories Second Year – Second Term Hematology – 1 Practice NO (3) Preparation of Haemolysates EDTA is the most convenient anticoagulant because it is used for the initial full blood count and film although samples taken into any anticoagulant are satisfactory. Cells freed from clotted blood can also be used. Preparation of Haemolysate for the Quantification of Haemoglobins and Stability Tests Preparation of haemolysate for the quantification of haemoglobins and stability tests can be used for qualitative electrophoresis and is necessary for quantitation of Hb A2 and F or variant haemoglobins by elution. It is also essential for reliable stability tests and globin electrophoresis. Lyse 2 volumes of washed packed cells in 1 volume of distilled water add 1 volume of carbon tetrachloride (CCl4). Alternatively, lyse by freezing and thawing, then add 2 volumes of CCl4. Shake the tubes vigorously for approximately 1 min then centrifuge at 1200 g (3000 rev/min) for 30 min at 4°C. Transfer the supernatant to a clean sample container and adjust the Hb to 100 ± 10 g/l with water. If an unstable Hb is suspected, organic solvents should be avoided Prepared By Dr Abdelrhman Elreshid 1 Practice NO (4) QUANTITATION OF Hb F Hb F may be estimated by several methods based on its resistance to denaturation at alkaline pH, by HPLC, or by an immunological method. Of the alkaline denaturation methods, that of Betke et al is reliable for small amounts (<10–15%) of Hb F, whereas for levels of more than 50%, and in cord blood, the method of Jonxis and Visser is preferable; however, this method is not reliable at levels of less than 10%. Immunological methods have been devised to measure Hb F by immunodiffusion, for which commercial kits are available, and by enzyme-linked immunoassay (ELISA). Modified Betke Method for the Estimation of Hb F Principle To measure the percentage of Hb F in a mixture of haemoglobins, sodium hydroxide is added to a lysate and, after a set time, denaturation is stopped by adding saturated ammonium sulphate. The ammonium sulphate lowers the pH and precipitates the denatured haemoglobin. After filtration, the quantity of undenatured (unprecipitated) haemoglobin is measured. The proportion of alkaliresistant (fetal) haemoglobin is then calculated as a percentage of the total amount of haemoglobin present. Equipment Filter paper. Whatman No. 42. Vortex mixer. Glass tubes. Prepared By Dr Abdelrhman Elreshid 2 Reagents Cyanide solution. Potassium cyanide, 25 mg; potassium ferricyanide, 100 mg. Dissolve in 500 ml distilled water. Store in a dark bottle. Saturated ammonium sulphate solution. Bring 1 litre of water to the boil and add ammonium sulphate until the solution is saturated. Cool and equilibrate at 20°C before use. 1.2 mol/l Sodium hydroxide. Sodium hydroxide 4.8 g; distilled water to 100 ml. Prepare monthly. Equilibrate at 20°C before use. Method 1. Prepare a lysate as described above. The lysate may be stored at 4°C for up to 1 week before use. 2. Add 0.25 ml lysate to 4.75 ml cyanide solution to make a solution of haemiglobincyanide (HiCN). 3. Transfer 2.8 ml of the haemiglobincyanide solution to a glass test tube and allow to equilibrate at 20°C. 4. Blow in 0.2 ml of 1.2 mol/l of NaOH and mix on a vortex mixer for 2–3 sec. 5. After exactly 2 min, blow in 2 ml saturated ammonium sulphate solution and mix on a vortex mixer. Leave tubes to stand for 5–10 min at 20°C. 6. Filter twice through the same Whatman No. 42 filter paper, using a clean test tube to collect the filtrate each time. If the filtrate is not completely clear, filter again through the same paper. This filtrate contains the alkali-resistant haemoglobin. 7. To measure the total haemoglobin, transfer 0.4 ml of the haemiglobincyanide solution from step 2 into another tube and add 13.9 ml of water. 8. Read the absorbance of the alkali-resistant and total haemoglobin at 420 nm against a water blank. 9. Calculate the percentage alkali-resistant haemoglobin as follows: Prepared By Dr Abdelrhman Elreshid 3 % Alkali resistant haemoglobin = A420 alkali - resistant Hb X 100 A420 total Hb X 20 Method of Jonxis and Visser Principle The increased resistance of Hb F to denaturation by alkali is detected by recording the change in absorption at 576 nm in each min, caused by the addition of ammonium hydroxide. At this wavelength, the absorption of oxyhaemoglobin differs from that of the alkali haemochromogen that is formed on denaturation. When the logarithm of the percentage of haemoglobin remaining undenatured is plotted against time, a straight line is obtained. By extrapolation to time zero, the percentage of Hb F in the original sample can be calculated. Reagents Ammonium hydroxide solution. NH4OH, 100 g, water to 1 litre. Sodium hydroxide solution 0.06 mol/l. Sodium hydroxide, 2.4 g, water to 1 litre. Method 1. All reagents should be allowed to reach room temperature before use. Add 0.1 ml of blood or lysate (100 g/l) to 10 ml of water and mix. 2. Add 2 drops of ammonium hydroxide solution and mix. 3. Measure the absorbance in a spectrophotometer at 576 nm (AB). 4. Add 0.1 ml of the same blood or lysate to 10 ml of sodium hydroxide solution; then add 2 drops of ammonium hydroxide solution and mix thoroughly. 5. Measure the absorbance in a spectrometer at 576 nm at every Prepared By Dr Abdelrhman Elreshid 4 min for 15 min (AT); then incubate the solution at 37°C for 15 min, cool to room temperature, and measure the absorbance (AE). The ratio AB:AE should be constant. 6. Calculate the percentage of undenatured haemoglobin at each min as follows: AT 576 - AE 576 X 100 AB 576 - AE 576 Plot the percentage on the logarithmic scale of semilogarithmic paper against time. This should produce a straight line from which the original amount of Hb F at time zero can be found by extrapolation. Interpretation and Comments The Jonxis and Visser method requires an accurate spectrometer because the maximum absorption peak at 576 nm is very narrow and the difference in extinction between oxyhaemoglobin and alkali haemochromogen is relatively small. Radial Immunodiffusion The radial immunodiffusion procedure can be used for the quantitation of Hb F. The principle is based on an antibody–antigen reaction; the anti-Hb F is incorporated into the gel support medium resulting in the formation of a visible opaque precipitin ring. The square of the diameter of this ring is directly proportional to the concentration of Hb F. A standard curve must be prepared from samples containing known levels of Hb F plotted against their haemoglobin concentrations. Interpretation of Hb F values Prepared By Dr Abdelrhman Elreshid 5 Hb F Range (%) Interpretation 0.2–1.0 Normal results 1.0–5.0 In approximately 30% of β thalassaemia traits Some heterozygotes for a variant haemoglobin Some homozygotes for a variant haemoglobin Some compound heterozygotes for a variant haemoglobin and β thalassaemia Some individuals with haematological disorders (aplastic anaemia, myelodysplastic syndromes, juvenile myelomonocytic leukaemia) Some pregnant women (second trimester) Sporadically in the general population, particularly in AfroCaribbeans (representing heterozygosity for nondeletional HPFH) 5.0–20.0 Occasional cases of β thalassaemia trait Some homozygotes for a variant haemoglobin Some compound heterozygotes for a variant haemoglobin and β thalassaemia Some types of heterozygous HPFH δβ thalassaemia 15.0– 45.0 Heterozygous HPFH African type (usually more than 20%) Some cases of β thalassaemia intermedia >45.0 β thalassaemia major Some cases of β thalassaemia intermedia Neonates >95.0 Homozygous African-type (deletional) HPFH Prepared By Dr Abdelrhman Elreshid 6 Hb F Range (%) Interpretation Some neonates (particularly if premature) Prepared By Dr Abdelrhman Elreshid 7