KHP Analysis: Acid-Base Titration Lab Experiment
advertisement
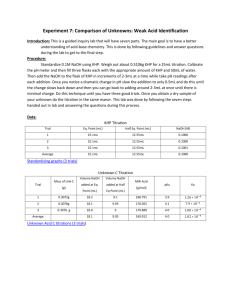
Chemistry 212 Experiment 3 ANALYSIS OF A SOLID MIXTURE LEARNING OBJECTIVES The objectives of this experiment are to - learn to analyze a solid unknown with volumetric techniques. - learn to use the LabWorks Interface for pH measurements. - determine the percentage of KHP in a solid mixture of KHP and a soluble salt. BACKGROUND In this experiment you will use an acid-base titration to determine the composition of a solid mixture, containing potassium hydrogen phthalate (KHP) and an inert, soluble salt (such as NaCl). The analysis will be carried out by weighing a portion of the unknown solid, dissolving it in water, and titrating the resulting solution with a standardized solution of sodium hydroxide. KHP is an organic weak acid with the structure which corresponds to formula KHC8H4O4. One of the hydrogen atoms in KHP is acidic and thus will react with a base such as OH-. When KHP is dissolved in water, it dissociates to produce potassium ions (K+) and hydrogen phthalate ions (HP-). When OH- is added to the solution, it extracts the acidic hydrogen atom from the HP- ion: HP- + OH- P2- + H2O 1 In this experiment, you will titrate the acid using the LabWorks interface to monitor pH. In the Analysis portion of the experiment, you will determine the equivalence point or stoichiometric point by monitoring the pH of the solution as base is added and then plotting a pH curve. The pH of the solution is a measure of the amount of hydrogen ion in the solution: pH = -log [H+] Hydrogen phthalate is a weak acid: HP- H+ + P2For pure KHP in water, the hydrogen ion concentration will be about 1.0 x 10-4 M which corresponds to a pH of about 4.0. The value actually observed depends on the concentration of HP- in solution. As base is added to a solution of KHP the HP- will be consumed and the pH of the solution will gradually increase. At first, when there is plenty of HP- available, the reaction of H+ with base will be offset by more dissociation of HP-, so there will be little change in the pH. This is called the buffering capacity of HP-. But when the HP- is nearly gone, the change in pH with the addition of OH- becomes greater. At the point where the HP- is completely consumed, the change in the pH will be the greatest. Consider the following graph of pH vs. mL of base added, (called a titration curve): At point E on the curve, the slope of the curve is greatest, since the pH is changing most rapidly in this area of the titration. This is the equivalence. Location of the equivalence point enables us to determine how many milliliters of base it took to exactly react with the HP- in solution. Because we also know the concentration of OH- in the base solution, we can calculate the number of moles and hence grams, of KHP in the original solid sample. The percent KHP (by mass) can then be calculated. 2 SAFETY PRECAUTIONS Safety goggles must be worn in the lab at all times. Any skin contacted with chemicals should be washed immediately. Calibration of the pH electrode 1. Attach the electrode to the interface unit at the pH/mV position and place the electrode in a buffer solution of pH 7. 2. Use the Calibrate choice on the LabWorks menu and choose pH. Follow the instructions to complete the calibration of your electrode. Enter the value of 7.00 to calibrate the electrode at a pH of 7.00. It is good technique to calibrate the electrode each time the computer is turned on. 3. Remove the electrode from the buffer solution and rinse it with deionized water. Design a LabWorks Experiment Go to Design and create the following experiment using the experiment builder option. Save the experiment file because you will be using the same data acquisition technique again in the future. Always test your experiment program before actually running the experiment. Calibration of Drop Size In this part of the experiment the volume of titrant will be determined by electronically counting the number of drops required to reach the equivalence point. Therefore, the volume of a single drop must be known accurately. It is important that the rate of delivery of the sodium hydroxide used in the calibration part of the experiment is the 3 same as that used during the actual titration. This ensures that the drop size remains constant. 1. Your instructor will demonstrate how to set up the drop counter. Align the counter by filling the buret with your NaOH solution and allowing it to drip through the counter into a small clean beaker. The buret is correctly aligned when the counter light on the interface flashes every time a drop falls from the buret. Alignment of the buret is a critical step in producing an accurate titration. Once aligned do not move the buret or serious errors will result. 2. Read the buret and record the initial volume. Go to the Acquire section and start the data acquisition program. Next, start the buret dripping at a moderately slow rate (1 or 2 drops per second). Let the program count drops for a minimum of 10 mL and then close the buret and click stop on the data acquisition screen. Record results and repeat. Divide the total volume by the number of drops to get the average dropsize. EXPERIMENTAL PROCEDURE You will do three determinations in this experiment Using the standardized NaOH solution (approx. 0.1 M), you will titrate the unknown mixture and determine the percentage of KHP. Titration of an Unknown Solid Mixture 1. Set up titration apparatus as demonstrated by your instructor. 2. Dissolve approximately 1.0 g (record the exact mass from the analytical balance on you data sheet) of the unknown solid mixture in about 70 mL of deionized water. Prepare two more mixtures, of the same unknown, in the same manner. 3. Titrate the first unknown solution with the standardized NaOH (NaOH solution is in the buret) using the LabWorks program you designed. 4. When the first titration is finished click stop and then save your data. Rinse the pH electrode with deionized water and repeat the experiment with the other two unknown solutions. 4 DATA ANALYSIS Determination of the Percent KHP in the Unknown Solid Mixture 1. In Analyze load the data from the titrations of your unknown acid. 2. In column D calculate the volume of NaOH added to the unknown solution. It can be calculated based on dropsize and drops counted. Formula =C1*dropsize 3. In column E calculate the derivative of pH with respect to time. This may help identify the equivalence point. Formula = @DERIV(B1,A1) 4. Calculate the percent KHP in the solid mixture based on the volume of NaOH needed to reach the equivalence point of the titration. (Your instructor will explain this calculation further.) 5 Data Sheet ANALYSIS OF A SOLID MIXTURE Calibration of drop size Volume Initial Volume Final Volume Total Number of Drops mL / Drop Ave.= Analysis of Unknown Solid Mixture - LabWorks Titration Unknown #__________ Concentration of NaOH___________moles\liter Trial 1 Trial 2 Mass Unknown (g) Vol. NaOH at Eq. (mL) Calc. Mass KHP (g) % KHP Show an example of your calculation to find mass of KHP in the unknown: 6 Trial 3 Report Sheet Name _____________________________ Partner(s)_____________________________________ Section ____________________________ Date ______________________________ Purpose of the experiment Results Unknown #__________ % KHP in Unknown Trial 1 Trial 2 Trial 3 Average Conclusion (Discuss results and be sure to include a discussion of precision and accuracy with a mention of random and systematic error.) 7