UNIT PLANNING TEMPLATE
advertisement

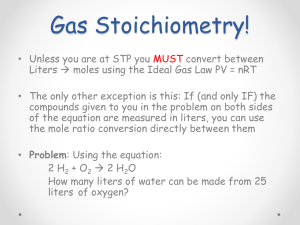
K1: CHEMISTRY UNIT OUTLINE UNIT K: MATHEMATICS OF CHEMICAL EQUATIONS: STOICHIOMETRY TOPICS: OBJECTIVES: FRAMEWORK STRANDS: LEARNING STANDARDS: CLASSROOM HANDOUT MATERIALS This unit is designed to teach you how to: use the coefficients from balanced chemical equations to derive information regarding moles, particles, relative volumes, and formula masses; use the above information to solve quantitative problems regarding reactants and products. Upon completion of this unit, you will be expected to: 1. list what information can derived from the coefficients of a balanced equation (moles, molecules/particles, liters, etc.), 2. determine the reltive number of moles, molecules/particles, and liters represented by the coefficients of a balanced equation, 3. convert the number of moles of a substance into sample mass, 4. convert the number of moles of a substance into molecules/particles, 5. convert the number of moles of a substance into liters, 6. use stoichiometric analysis and dimensional analysis (factor label method) to solve problems involving the calculation of the mass, moles, particles and/or liters of a product given quantitative information about a reactant, 7. use stoichiometric analysis and dimensional analysis (factor label method) to solve problems involving the calculation of the mass, moles, particles and/or volume (in mL or L) of a product given quantitative information about a product. CHEMISTRY CH.1g, CH.3d, CH.4a, b K1: UNIT OUTLINE B2: PERIODIC TABLE F3: Naming Compounds Flowchart K2: BROWNIE STOICHIOMETRY K4: #K-4 W assignment Stoichiometry Practice Problems/Unit K Study Guide K5: Unit K Pre-test K6: Unit K Notes VOCABULARY/ You are expected to be thoroughly familiar with the following. You will find CONCEPTS/ definitions, explanations, and information about these in your reading (reference SCIENTISTS sections are noted). They will also be explained, discussed, and illustrated through classroom and lab activities. K1-1 composition stoichiometry and reaction stoichiometry (9-1) K1-2 types of reaction stoichiometry problems (9-1) K1-3 Avogadro’s Hypothesis (Law) (12-2) K1-4 STP (12-2) K1-5 molar volume of gases (12-2) K1-6 what coefficients can represent (mole ratios, volume ratios, molecule ratios) (9-1, unit notes) K1-7 volume—volume problems (unit notes) K1-8 stoichiometry using a recipe (unit notes) K2-1 K2-2 procedure for solving mass—mass problems (9-1, unit notes) procedure for solving mixed mass—volume—particle problems (9-1, unit notes) K3-1 K3-2 limiting and excess reactants (9-2) reasons for using excess reactants in real life (9-2) K3-3 K3-4 determining the limiting reactant, and calculating product yields(9-2) percent yield (9-2) EVALUATION: ASSIGNMENTS: #K-1 Reading: Written: You will be evaluated via the following: Daily homework quizzes based on reading and written assignments Unit Quiz—covers all objectives stated above. 9-1 pp. 303-305, 12-2 (pp. 431-432 only), K-1 R notes p. 304: PP#2; p. 309 # 3, 4; p. 330 #29 (c), 30 (c) (make sure to write the relative mole, particle, or volume recipe for each balanced equation, and don’t worry about density values given in the problem statement) #K-2 Reading Written 9-1 (pp. 306-311), K-2 R notes A: p. 311: # 4 (all), 5 (a), 7 (What is the reaction type in problems 4, and 5?) B: p. 330 #24, 27, 29 (assume reaction conditions are @ STP, and don’t worry about density values given in the problem statement—remember to convert between L and mL) #K-3 Reading Written 9-2 , K-3 R notes A: p. 331: # 32, 33, 37, 38 B: p. 319: # 8, 9 (what type of reaction is this? Remember to write and balance the equation first!), 11, 12 #K-4 Written Stoichiometry Practice problems worksheet