nucleophillic aromatic substitution reactions
advertisement
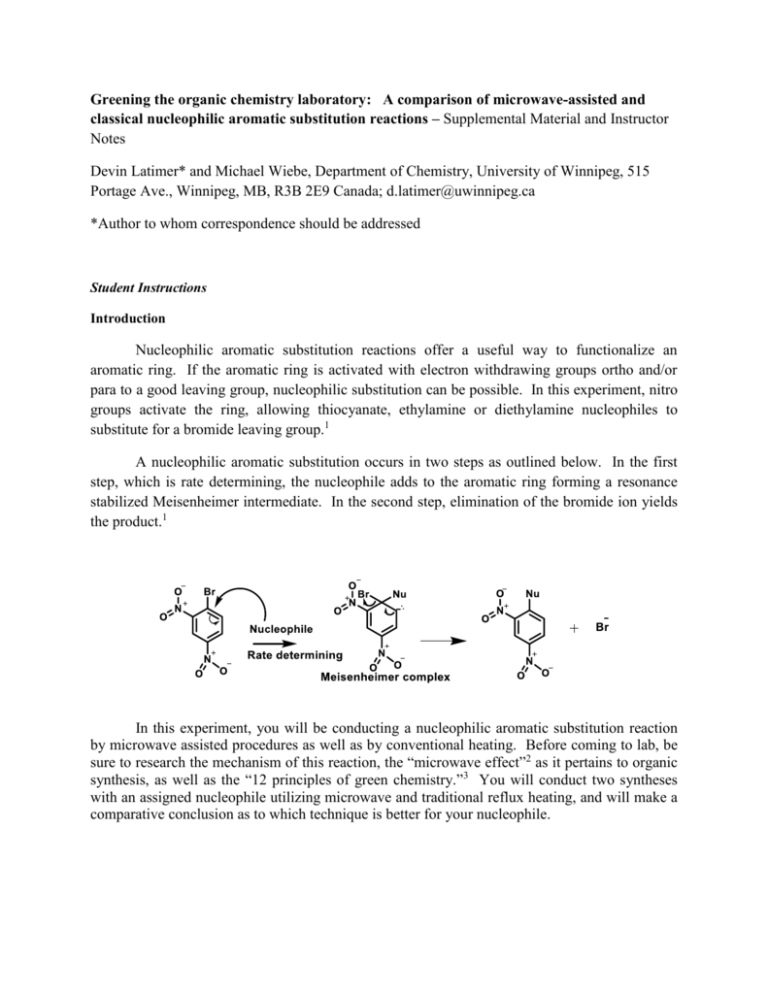
Greening the organic chemistry laboratory: A comparison of microwave-assisted and classical nucleophilic aromatic substitution reactions – Supplemental Material and Instructor Notes Devin Latimer* and Michael Wiebe, Department of Chemistry, University of Winnipeg, 515 Portage Ave., Winnipeg, MB, R3B 2E9 Canada; d.latimer@uwinnipeg.ca *Author to whom correspondence should be addressed Student Instructions Introduction Nucleophilic aromatic substitution reactions offer a useful way to functionalize an aromatic ring. If the aromatic ring is activated with electron withdrawing groups ortho and/or para to a good leaving group, nucleophilic substitution can be possible. In this experiment, nitro groups activate the ring, allowing thiocyanate, ethylamine or diethylamine nucleophiles to substitute for a bromide leaving group.1 A nucleophilic aromatic substitution occurs in two steps as outlined below. In the first step, which is rate determining, the nucleophile adds to the aromatic ring forming a resonance stabilized Meisenheimer intermediate. In the second step, elimination of the bromide ion yields the product.1 In this experiment, you will be conducting a nucleophilic aromatic substitution reaction by microwave assisted procedures as well as by conventional heating. Before coming to lab, be sure to research the mechanism of this reaction, the “microwave effect”2 as it pertains to organic synthesis, as well as the “12 principles of green chemistry.”3 You will conduct two syntheses with an assigned nucleophile utilizing microwave and traditional reflux heating, and will make a comparative conclusion as to which technique is better for your nucleophile. Pre-lab questions 1. What is meant by the term microwave effect? Predict the optimal synthetic route (microwave or conventional heating) for your reaction. 2. Why is an excess of nucleophile used in each reaction? 3. Draw each resonance form of the intermediate Meisenheimer complex. 4. What are the 12 principles of green chemistry? Student Procedure (microwave reactions)2 In a 10 mL glass microwave vessel mix 0.298 g (1.2 mmol) of 1-bromo-2,4dinitrobenzene with your assigned nucleophile (0.467 grams of potassium thiocyanate, 0.38 mL 70% aqueous ethylamine (d = 0.796 g/mL) in 3 mL of ethanol or 0.27 mL of 99.5 % diethylamine (d = 0.704 g/mL) in 3 mL of ethanol) and 2.5 mL ethanol and 0.5 mL water. [CAUTION: refer to hazards section, wearing gloves is mandatory.] Add a stir bar and cap the reaction vessel with a microwave plastic cap to prevent hot solvent from damaging the microwave vessel. Note that the reactants will not dissolve completely in the solvent system until heated in the microwave. Using a metal attenuator of the correct size (the glass vessel should be able to fit securely) and secure the capped reaction vessel in the microwave cavity of the CEM DISCOVER SP microwave reactor. 1. Note: Ensure that the microwave vessel fits securely inside the glass attenuator, there should be a plastic cup as well as a metal piece in the bottom of the microwave for the tube to rest inside. After inspecting, firmly turn the metal hinges to lock the glass tube in place (they will only turn in 1 direction) until a click is heard; the microwave will not start unless the vessel is locked in place 2. Above: starting screen displayed on the microwave unit upon it starting up; select “user” using the arrow keys, and once highlighted as shown in the diagram, hit the enter or large arrow key in the middle of the console 3. Next, press the Discover key (with a large D on it) and the screen to the left should be displayed. Using the arrow keys, select SPS (standard pressure setting) and press the enter key. The screen should then appear as shown on the right 4. Press the “T” key to bring up the screen shown on the left and using the arrow keys (up, down, left and right), set the parameters to a microwave energy of 150 Watts, time of 5 minutes 30 seconds, temperature of 125 degree Celsius and delta temperature of 3 degrees Celsius. 5. When all of the parameters are set, and the screen above is still displayed, press the play button to start the reaction. The microwave will automatically cover the glass tube and start the reaction (shown above). DO NOT press anything during the reaction, the reaction will run and subsequently cool to about 60 0 C and automatically uncover your reaction; please do not interrupt either the reaction or the cooling cycle 6. Once the reaction is completed and the reactor has cooled to about 60 degrees Celsius (this will take about 7 minutes), the cover will automatically retract and you can remove your reaction tube. Transfer the cooled reaction vessel into an ice bath to begin crystallization. Filter the precipitate by vacuum filtration using a Hirsch funnel and wash with cold ethanol. Weigh and calculate the percent yield for your reaction and remove a small portion of the crude product to dry on a watch glass for characterization. Transfer the remainder of your crude product into a clean 25 mL Erlenmeyer flask and recrystallize from 3-5 mL ethanol (30-35 mL ethanol for the thiocyanate reaction). Conduct a semi-micro filtration of the hot solution through a pipette using glass wool (this may require keeping the solution on the sand bath and near the boiling point during the filtration as slight cooling may result in precipitation and product loss). Allow to crystallize gradually on ice to 0 o C and collect by suction filtration. Allow your product to dry and confirm its identity by melting point, 13C NMR spectroscopy and mass spectrometry. You will be provided with the spectra of the reactant (1-bromo-2-4 dinitrobenzene) to aid in your analysis. Student procedure (reflux reactions)4 In a fume hood, assemble a small 10 mL round bottom containing a magnetic spin vane and equipped with a reflux condenser. Weigh and place in the vial 0.298 g (1.20 mmol) of 2,4dinitrobromobenzene, followed by 2.1 mL of toluene. Add 0.030 g (0.10 mmol) of tetrabutylammonium bromide and your assigned nucleophile (0.93 mL of a 50% aqueous potassium thiocyanate (wt/wt) solution, 0.38 mL 70% aqueous ethylamine (d = 0.796 g/mL) in 3 mL of ethanol or 0.27 mL of 99.5 % diethylamine (d = 0.704 g/mL). [CAUTION: refer to hazards section, wearing gloves is mandatory.] Heat the resulting mixture to a temperature of 110°C, with stirring for 1 hour and allow the solution to cool to room temperature. For best results do not allow the solution to reflux for longer than one hour. Separate the organic and aqueous layers. Wash the organic toluene layer with two portions of water (6 mL each) and then extract the combined aqueous washes with two portions of toluene (6 mL each). Dry the combined organic layers over anhydrous magnesium sulfate for 5 minutes, filter and concentrate the solution using a rotary evaporator set at 50-60 oC. Weigh and calculate the percent yield for your reaction and put a small amount aside to determine the melting point of your crude product and recrystallize from ethanol as described in the microwave procedure above. Allow to dry before characterization of the final product. Liquid and solid waste generated during the experiment should be disposed of in the appropriate organic waste containers. Post-lab Considerations: (1) Given the similarity in appearance of the reactant and product compounds and their respective structures, describe two different characterization techniques you could use to determine the success of the reaction. This may require a literature reference as part of your explanation. (2) State one characterization technique that would not be as effective as a technique listed in (1). (3) Based on your results (time involved, % yield, solvents and other materials used), which of the techniques (microwave-based synthesis or reflux synthesis) do you think performed “better” for your particular nucleophile in this reaction. Provide some green metric calculations, such as atom economies and E-factors, and discussions of other synthetic parameters for each technique and argue which is “greener”. [For a detailed example of these parameters, please see the discussion in section 1.4 of the text by Dicks5] In your lab report, be sure to speculate on what other parameters of the experiment might be developed to lead to a greener synthesis, and outline how one could conduct a study on these parameters. Analysis of Results Fill in the table below with yield and other notes/comments for each reaction using personal and class results. You are required to find physical and spectral properties from literature sources and compare with your own data to determine the success of your reaction. Compare the techniques used for each chosen nucleophile. Reaction Yields and Metrics Substrate Nucleophile Condition Microwave irradiation Reflux Percent Yield, Atom Economy, Overall Reaction Efficiency Other green chemistry metrics to be discussed Melting Point, Spectra Obtained Microwave irradiation Reflux Microwave irradiation Reflux Hazards Gloves should be worn when handling ethylamine, diethylamine, potassium thiocyante and tetrabutylammonium bromide as they are toxic upon skin contact. Ethanol, ethyl acetate and toluene are toxic and flammable and should be handled with care. The reactant, 1-bromo-2,4dinitrobenzene is both toxic and flammable and the synthesized 2,4-dinitrophenyl thiocyanate product is also toxic upon skin contact. The products of the other two reactions, 2,4-dinitro-N-ethylaniline and 2, 4-dinitro-N,Ndiethylaniline are both skin and respiratory tract irritants, handle with care. The d6-acetone used as a NMR solvent is a skin and eye irritant and causes chronic organ toxicity upon extended exposure. As a general precaution, safety glasses, lab coats as well as gloves are recommended for the duration of the laboratory exercise. Students are advised to avoid skin contact and inhalation of the chemicals used and to employ a fume hood while adding ethylamine and diethylamine solvents to their reaction vessels. Material safety data sheets are available from Sigma Aldrich should emergency measures be required; it is highly recommended that students refer to the MSDS before beginning any laboratory experiment. In addition, caution should be exercised when using the microwave reactor. The microwave reactor should be allowed to cool before venting to prevent damage to the microwave cavity. Notes for the Instructor - - - - - - - Before microwave heating, reagents are insoluble in the solvent systems. Upon microwave or conventional heating, the reactants will dissolve, and will subsequently precipitate as the reaction is cooled. The microwave reactor should be allowed to self-cool before the cavity is opened. Manual termination of the cooling cycle could damage the microwave cavity as the pressure buildup from the solvent is vented Removal of toluene solvent should be done at moderate temperature (50-60 oC) on a rotoevaporator (or similar reduced pressure) to avoid product decomposition. During the ethanol recrystallization, the diethylamine and ethylamine products display a greater solubility in the solvent than the product of the potassium thiocyanate reaction. Approximately 3-5 mL of solvent should be used to effect the purification of these products. Methods of product characterization are at the instructor’s discretion. The most definitive techniques are, perhaps, the combination of 13C NMR, mass spectrometry and melting point determination that we present here. See attached figures for examples of student product spectra. Students are to assign peaks in the product spectra, limited to comparison with the reactant 1-bromo-2,4-dinitrobenzene spectra which are given as handouts. For example, students note the 6 individual peaks in the 13C NMR spectrum of 1-bromo-2,4-dinitrobenzene and then the presence of the seventh peak in the sphybridized region for the thiocyanate group in the spectrum of 13C NMR spectrum of 2,4dinitrophenylthiocyanate. Students are encouraged to look through the chemistry literature in order to compare physical and spectral properties of their product. In particular, an introduction to the use of SciFinder would be an asset. Melting points of the reactant and products are: o 2,4-dinitrobromobenzene: 71-73 oC2 o 2,4- dinitrophenyl thiocyanate: 139-141 oC2 o 2,4-dinitro-N-ethylaniline: 113-114 oC6 o 2,4-dinitro-N,N-diethylaniline: 80 oC7 From preliminary data, the authors note that ethanol can also be used as a reflux solvent (following reagent quantities used in the microwave procedure) resulting in similar yields to the toluene/TBAB system. Students are advised not to extend the reflux time beyond one hour as it seems to complicate the reaction work-up, especially for the diethylamine reaction. Equipment Required (per 10 students): - - - CEM Discover SP microwave or other microwave reactor (placed in a fume hood) 10 Micro-stir bars, (10) 10 mL glass microwave vessels and (10) disposable microwave caps supplied by CEM or other microwave supplier - one of each item per student plus extras Small metal microwave attenuator (just large enough to fit the 10 mL glass vessel) a syringe or micro-pipette to measure 0.38 mL of ethylamine and diethylamine A constant supply of ice for recrystallization Hot plates for reflux reactions and recrystallization (10) reflux condensers (10) 10 mL round bottom flasks (10) 25 mL Erlenmeyer flasks for recrystallizations (10-20) Small 3 mL glass sample vials for storing products (10) Hirsch funnel + gaskets (1 per student) (10) 125 or 250 mL filter flasks with rubber tubing 1 box of Hirsch funnel filter paper Weighing papers Sand bath and (5-10) hot plates for recrystallization Glass wool for filtrations Pasteur pipettes and bulbs (2) Mel temp apparatus and capillary tubes to determine melting point For NMR, d6-acetone and NMR tubes will be needed for characterization of product, 13C NMR spectra attached here were obtained using a 400 MHz Bruker nuclear magnetic resonance spectrometer equipped with a BioSpin Ultra Shield Magnet with Avance™ III NanoBay system. GC-MSD mass spectra attached here were acquired on a Hewlett-Packard Gas Chromatograph/Mass Selective Detector (5970 Series) [Column: Agilent HP-5MS, 30 m x 0.250 mm x 0.50 um (part# 19091S-133). Injection port temperature: 250 0C. Detector temperature: 280 0C. Oven temperature is set to 50 0C, with a 20 oC/minute ramp to final temp of 210 0C. Flow rate is set to 35 mL/minute with split flow of 2 mL/minute.] Chemicals and Reagent amounts required (per approximately 10 students): Chemicals were obtained from Sigma Aldrich and used without further purification, solvents were supplied by EMD chemicals. CAS numbers are in parentheses beside each chemical. - 5 grams KSCN (330-20-0) - 10 mL 68-72% ethylamine (75-04-7) - 5 grams 1-bromo-2,4-dinitrobenzene (584-48-5) - 10 mL diethylamine (109-89-7) - 5 grams tetrabutylammonium bromide (1643-19-2) - 500 mL ethanol (64-17-5) - 50 mL ethyl acetate (solvent for mass spectrometry) (141-78-6) - 25 mL toluene (108-88-3) - 20 mL d6-acetone (666-52-4) Supporting Spectroscopic data C NMR spectral data were obtained using a 400 MHz Bruker nuclear magnetic resonance spectrometer equipped with a BioSpin Ultra Shield Magnet with Avance™ III NanoBay system. All of the following spectra are student generated. GC-MSD mass spectra were acquired on a Hewlett-Packard Gas Chromatograph/Mass Selective Detector (5970 Series) [Column: Agilent HP-5MS, 30 m x 0.250 mm x 0.50 um (part# 19091S-133). Injection port temperature: 250 0C. Detector temperature: 280 0C. Oven temperature is set to 50 0C, with a 20 0C/minute ramp to final temp of 210 0C. Flow rate is set to 35 mL/minute with split flow of 2 mL/minute.] 13 A) 1-bromo-2-4 dinitrobenzene Figure A-1: 1H-decoupled 13C NMR spectrum of the reactant compound 1-bromo-2,4dinitrobenzene in d6-acetone. Figure A-2: GC-MSD mass spectrum of the reactant compound 1-bromo-2,4-dinitrobenzene in ethyl acetate. Compound was observed at a retention time of 12.703 minutes. B) 2,4- dinitrophenyl thiocyanate Figure B-1: 1H-decoupled thiocyanate in d6-acetone. 13 C NMR spectrum of the product compound 2,4-dinitrophenyl Figure B-2: GC-MSD mass spectrum of the product compound 2,4-dinitrophenylthiocyanate in ethyl acetate. Compound was observed at a retention time of 16.43 minutes. C) 2,4-dinitro-N-ethylaniline Figure C-1: 13C attached proton (APT) NMR spectrum of the product compound 2,4-dinitro-Nethylaniline in d6-acetone. Carbons with even and odd numbers of hydrogen atoms are displayed above and below the spectral axis respectively. Figure C-2: GC-MSD mass spectrum of the product compound 2,4-dinitro-N-ethylaniline in ethyl acetate. Compound was observed at a retention time of 19.293 minutes. D) 2,4-dinitro-N,N-diethylaniline Figure D-1: 1H-coupled 13C NMR spectrum of the product compound 2,4-dinitro-N,Ndiethylaniline in d6-acetone. Figure D-2: GC-MSD mass spectrum of the product compound 2, 4-dinitro-N-N-diethylaniline in ethyl acetate. Compound was observed at a retention time of 19.002 minutes displayed above. Acknowledgements The authors wish to thank Peter Balagus (microwave reactor and GC-MS) and Ramin Vakili (NMR) for valuable contributions to this study. References (1) Brown, W. H.; Foote, C. S.; Iverson, B. L.; Anslyn, E. V.; Novak, B. M. Organic Chemistry, New York, 2011. (2) Leadbeater, N.; McGowan, C.; Clean Fast Organic Chemistry, CEM Publishing, Matthews, 2006, 23-25. (3) Anastas, P.; Warner, J. Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998. (4) Mayo, D. W.; Pike, R. M.; Trumper, P. K. Microscale Organic Laboratory, 4 th edition; John Wiley and Sons, Inc.; New York , 2008; pp. 345-349. (5) Dicks, A. P. Green Organic Chemistry in Lecture and Laboratory, CRC Press, Boca Raton, USA, 2012, 5-22. (6) Lamm, B. Acta. Chem. Scand., 1965, 19, 2316-2322. (7) Plakidin, V.; Vostrova, V. Zhurnal Organicheskoi Khimli, 1982, 18, 342-352.