moles iron and moles copper
advertisement
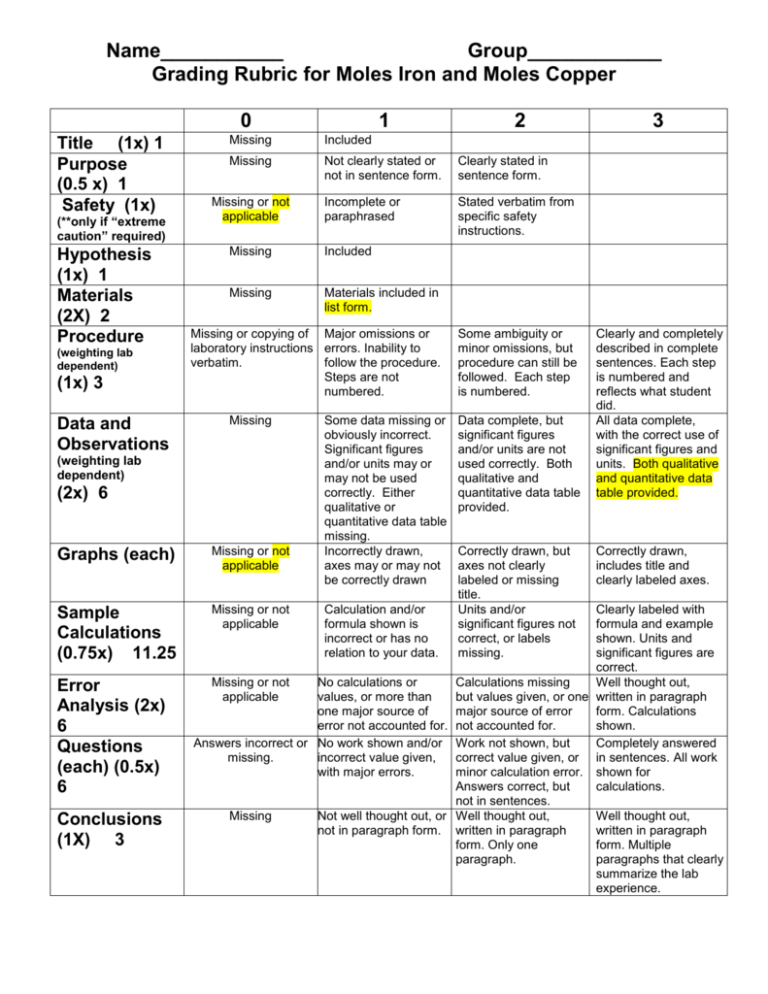
Name___________ Group____________ Grading Rubric for Moles Iron and Moles Copper 0 Title (1x) 1 Purpose (0.5 x) 1 Safety (1x) (**only if “extreme caution” required) Hypothesis (1x) 1 Materials (2X) 2 Procedure (weighting lab dependent) (1x) 3 Data and Observations Missing Not clearly stated or not in sentence form. Clearly stated in sentence form. Incomplete or paraphrased Stated verbatim from specific safety instructions. Missing or not applicable Missing Included Missing Materials included in list form. Missing or copying of Major omissions or laboratory instructions errors. Inability to verbatim. follow the procedure. Steps are not numbered. Missing (2x) 6 Graphs (each) Missing or not applicable Sample Calculations (0.75x) 11.25 Missing or not applicable Conclusions (1X) 3 2 Included (weighting lab dependent) Error Analysis (2x) 6 Questions (each) (0.5x) 6 1 Missing Some data missing or obviously incorrect. Significant figures and/or units may or may not be used correctly. Either qualitative or quantitative data table missing. Incorrectly drawn, axes may or may not be correctly drawn Calculation and/or formula shown is incorrect or has no relation to your data. Some ambiguity or minor omissions, but procedure can still be followed. Each step is numbered. Data complete, but significant figures and/or units are not used correctly. Both qualitative and quantitative data table provided. Correctly drawn, but axes not clearly labeled or missing title. Units and/or significant figures not correct, or labels missing. 3 Clearly and completely described in complete sentences. Each step is numbered and reflects what student did. All data complete, with the correct use of significant figures and units. Both qualitative and quantitative data table provided. Correctly drawn, includes title and clearly labeled axes. Clearly labeled with formula and example shown. Units and significant figures are correct. Missing or not No calculations or Calculations missing Well thought out, applicable values, or more than but values given, or one written in paragraph one major source of major source of error form. Calculations error not accounted for. not accounted for. shown. Answers incorrect or No work shown and/or Work not shown, but Completely answered missing. incorrect value given, correct value given, or in sentences. All work with major errors. minor calculation error. shown for Answers correct, but calculations. not in sentences. Missing Not well thought out, or Well thought out, Well thought out, not in paragraph form. written in paragraph written in paragraph form. Only one form. Multiple paragraph. paragraphs that clearly summarize the lab experience. Calculations: Calculation 1. Mass of iron lost by the nails 1. Number of moles of iron used in the reaction 2. Mass of the solid copper product 3. Number of moles of copper produced 4. Mole ratio of iron used to copper produced. (Expressed as whole-number ratio) 5. Theoretical mass copper Error analysis section: Error (copper) % Error (copper) Error analysis: Please provide the calculations for and discussion regarding amount of copper collected. Questions: 1. a. What evidence do you have that a chemical reaction occurred? b. How do you know iron was used in the reaction? c. How do you know copper was produced in the reaction? 2. Explain how you experimentally determined the mole ratio of iron:copper. 3. How can you use your experimental mole ratio to determine the correct balanced chemical equation for the reaction between iron and copper (II) chloride? 4. Please provide the two balanced chemical equations (as determined in class) and then determine the mass of copper that should have been produced. [mass : mass] Conclusion: (As per rubric)