MLT 230 Urinalysis and Body Fluids
advertisement

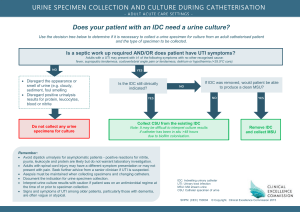
-CATALOGUE #MLT 230 CIP #51.1004 DATE: February 1, 2008 Moberly Area Community College COMMON SYLLABUS MLT: 230: Urinalysis and Body Fluids Current Term Instructor: Office Number: Office Hours: Contact information: Course description: MLT 230 Urinalysis and Body Fluids (w/clinicals) (1–4–2) The course covers various aspects of urinalysis including terminology, physical, microscopic and chemical exam, and current screening tests offered by laboratories. The course also studies various body fluids and associated analysis techniques. The student will learn and perform various tests and analysis procedures employed by laboratories. Prerequisites: BOE 171 Medical Terminology, BIO 205 Human Anatomy, BIO 209 Physiology and MLT 150 Laboratory Methods and Management. Text: Brunzel. Fundamentals of Urine and Body Fluid Analysis. 3rd Edition. Davis Publisher. ISBN: 978-1-4377-0989-6. Other Required Materials: Handouts, videos, training aids, and transparencies as provided by the instructor. Purpose of the course: The purpose of this course is to provide comprehensive instruction in the analysis body fluids, not including blood. In order to do this instructional material covers renal structure and function, urine preanalytical, analytical, and postanalytical factors, renal diseases, and metabolic disorders involving urine. The course moves on to study analysis of cerebrospinal fluid, semen, synovial fluid, serous fluid, amniotic fluid and fecal analysis. Cognitive Objectives: It is the responsibility of the student to learn the following material presented in Urinalysis and Body Fluid lectures, handouts, textbooks, audiovisuals, and computer-assisted learning: 1. Describe safe handling of urine and body fluids and acceptable methods of disposal. 2. Identify the components of the nephron, kidney, and excretory system. 3. Trace blood flow through the nephron and explain physiologic processes that occur. 2/17/2016 1 4. Explain glomerular filtration and the feedback mechanisms of renin-angiotensinaldosterone. 5. Discuss the process of tubular reabsorption including the mechanisms of active and passive transport and the activities of antidiuretic hormone. 6. Describe tubular secretion and, importantly, the part it plays in maintaining acidbase balance. 7. Discuss the laboratory procedures performed to measure glomerular filtration, tubular reabsorption and secretion, and renal blood flow. 8. Identify organic and inorganic components in urine. 9. Compare types of urine specimens encountered in medical analysis. 10. Discuss urine specimen collection, handling, preservation, and storage. 11. Identify and discuss the components analyzed in the physical examination of urine specimen. 12. Describe and discuss the chemical, confirmatory, and supplementary tests in urinalyses. 13. Describe the false positive reactions that could occur using urine reagent strips and possible sources of error. 14. List and identify formed elements in urinary sediment. 15. Define specific gravity, discuss how it is measured, and reasons for corrections. 16. List the analytical effects on a urine specimen kept at room temperature for longer than two hours. 17. Correlate physical and chemical urinalysis results with microscopic findings and recognize discrepancies. 18. Discuss the quality control procedures and documentation for quality control of urine and body fluids. 19. List the elements required for quality assurance as regulated by the Clinical Laboratory Improvement Amendments (CLIA ’88). 20. Differentiate disorders involving renal function and summarize primary urinalysis findings resulting from them. 21. Identify and discuss urine screening tests for metabolic disorders. 22. Discuss Cerebrospinal Spinal Fluid analysis: Function, specimen types, handling, analysis, pathologic conditions requiring the examination of, types of cells seen during examination of, chemical components. 23. Describe a semen specimen and discuss analysis of it. 24. Describe synovial fluid, physical aspects, normal and abnormal laboratory findings, and correlations with disease states. 25. Discuss laboratory examination of serous fluids, including appearance, chemistry, hematology, microbiology, and serology tests. 26. Discuss laboratory examination of amniotic fluid, including specimen collection, handling, appearance, analyses for pathologic conditions. 27. Discuss tests performed in fecal analysis and pathologic conditions requiring testing of feces. Psychomotor Objectives: Having instruction and practice in the student lab and at the affiliate site, students are responsible for performing the following to minimal competency standards: 2/17/2016 2 1. Instruct patients on the proper clean catch urine collection. 2. Evaluate specimen quality, describe corrective action to solve problems with improper specimens, and correctly process specimens for testing. 3. Follow laboratory policies for record keeping and reporting. 4. Perform daily maintenance routines. 5. Perform quality control and document values for urinalyses and body fluid testing. 6. Competently conduct physical, chemical, and microscopic examinations of urine specimens. 7. Describe and discuss abnormal findings when conducting physical, chemical, and microscopic examinations of urine specimens. 8. Competently perform confirmatory and supplementary urine tests. 9. Competently perform CSF manual cell count and differential count. 10. Identify cells and perform glucose testing in body fluids such as synovial and serous fluids. 11. Competently operate instrumentation for urinalyses. 12. Troubleshoot procedures and take appropriate action as needed. Affective Objectives: By the end of the course, the student is responsible for demonstrating the following behaviors and attitudes: 1. Perform urinalyses and body fluid testing with good technique, carefully with attention to detail and quality. 2. Maintain and operate equipment and instrumentation reliably; generate quality results. 3. Leave work area, equipment and instrumentation clear and in good working order after use. 4. Follow written procedures. 5. Organize for priority and efficiency. 6. Strictly follow written procedures and verbal instructions. 7. Use proper quality control measures. 8. Adhere to confidentiality policies. 9. Demonstrate initiative and resourcefulness; learn more than the minimum. 10. Exhibit a professional appearance and attitude. 11. Communicate with instructors, other healthcare professionals, and patients in a clear professional way. Course Outline: I. Renal Function A. Renal Physiology 1. Renal Blood Blow 2. Glomerular filtration 3. Tubular reabsorption 4. Tubular secretion B. Renal Functions Tests 2/17/2016 3 II. III. IV. 1. Clearance tests 2. Reabsorption tests 3. Tubular secretion and renal blood flow tests Urine Specimen A. Safe handling B. Composition C. Volume D. Specimen collection E. Specimen handling 1. Specimen integrity 2. Specimen preservation F. Types of Specimens 1. Random 2. First morning 3. Fasting 4. 2-hr. postprandial 5. Glucose tolerance 6. 24-hr. 7. Catheterized 8. Midstream clean-catch 9. Suprapubic aspiration 10. Three-glass 11. Pediatric 12. Drug collection Physical Examination of Urine A. Color 1. Normal 2. Abnormal a. Dark yellow/amber/orange b. Red/pink/brown c. Brown/black d. Blue/green B. Clarity 1. Normal 2. Nonpathologic turbidity 3. Pathologic turbidity C. Specific gravity 1. Urinometer 2. Refractometer 3. Harmonic oscillation densitimetry 4. Clinical correlations D. Odor Chemical Examination of Urine A. Reagent strips 1. Technique 2. Handling and storage 2/17/2016 4 3. Quality control 4. pH a. Clinical significance b. Reagent strip reactions 5. Protein a. Clinical significance b. Prerenal proteinuria c. Bence Jones d. Renal proteinuria e. Orthostatic (postural) proteinuria f. Microalbuminuria g. Postrenal proteinuria h. Reagent strip reactions i. Reaction interference j. Sulfosalicylic acid precipitation test 6. Glucose a. Clinical significance b. Reagent strip (glucose oxidase) reactions c. Reaction interference d. Copper reduction test e. Comparison of glucose oxidase and Clinitest 7. Ketones a. Clinical significance b. Reagent strip reactions c. Reaction interference 8. Blood a. Clinical significance b. Hematuria c. Hemoglobinuria d. Myoglobinuria e. Reagent strip reactions f. Reaction interference 9. Bilirubin a. Bilirubin production b. Clinical significance c. Reagent strip (diazo) reactions d. Reaction interference 10. Urobilinogen a. Clinical significance b. Reagent strip reactions and interference c. Reaction interference d. Ehrlich’s tube test e. Watson-Schwartz differentiation test f. Hoesch screening test for porphobilinogen 11. Nitrite a. Clinical significance 2/17/2016 5 V. VI. VII. VIII. b. Reagent strip reactions c. Reaction interference 12. Leukocyte esterase a. Clinical significance b. Reagent strip reaction c. Reaction interference 13. Specific gravity a. Reagent strip reaction b. Reaction interference Microscopic Examination of the Urine A. Macroscopic screening B. Preparation and examination of the sediment C. Sediment examination techniques D. Sediment constituents 1. RBC 2. WBC 3. Epithelial cells 4. Fat bodies 5. Bacteria 6. Yeast 7. Parasites 8. Sperm 9. Mucus 10. Casts 11. Crystals Quality Assurance A. Procedure manual B. Preanalytical factors C. Analytical factors D. Postanalytical factors E. CLIA ‘88 Renal disease A. Glomerular disorders 1. Glomerulonephritis 2. Vasculitis 3. Immunoglobulin A nephropathy 4. Nephrotic syndrome B. Tubular disorders 1. Hereditary 2. Metabolic C. Interstitial disorders 1. Pyelonephritis D. Vascular disorders E. Renal failure F. Renal calculi Metabolic disorders 2/17/2016 6 IX. X. XI. A. Overflow B. Amino acid disorders 1. Phenylalanine-tyrosine disorders 2. Branched-chain amino acid disorders 3. Tryptophan disorders 4. Cystine disorders C. Porphyrin disorders D. Mucopolysaccharide disorders E. Purine disorders F. Carbohydrate disorders Cerebrospinal fluid A. Formation and physiology B. Specimen collection and handling 1. Appearance 2. Traumatic collection C. Cell count 1. Methodology 2. Total cell count 3. WBC count 4. Corrections for contamination 5. Quality control D. Differential count 1. Cytocentrifugation 2. Cells E. Chemistry tests 1. Protein 2. Glucose 3. Lactate 4. Glutamine 5. Enzymes F. Microbiology tests 1. Gram stain and other stains 2. Latex agglutination G. Serologic testing Semen A. Physiology B. Specimen collection and handling C. Semen analysis D. Motility E. Morphology Synovial Fluid A. Physiology B. Specimen collection and handling C. Appearance and viscosity D. Cell counts E. Differential count 2/17/2016 7 F. Crystal identification G. Chemistry tests H. Microbiology and serology XII. Serous fluid A. Formation B. Specimen collection and handling C. General laboratory procedures 1. Pleural fluid 2. Pericardial fluid 3. Peritoneal fluid XIII. Amniotic fluid A. Physiology B. Specimen collection, processing, and handling C. Color and appearance D. Tests for fetal distress E. Tests for fetal maturity XIV. Fecal analysis A. Physiology B. Specimen collection C. Macroscopic screening D. Microscopic examination 1. Fecal leukocytes 2. Muscle fibers 3. Fecal fat E. Chemical testing 1. Occult blood 2. Quantitative fecal fat 3. Fetal hemoglobin (APT) XV. Automation Student Laboratory/Clinical Schedule: Students will spend some student laboratory time performing urinalyses, supplementary testing, and working with selected body fluids. In many laboratories urinalysis is performed in hematology; however, if it is performed in another laboratory area, students are advised to document laboratory test experience wherever they acquire it. Urinalysis and Body Fluids -Student Laboratory Activities: Having instruction and practice in the student laboratory, students are responsible for learning to perform the following: 1. Correctly perform and interpret urine reagent strip chemistries. 2. Construct a table listing the causes of false positive and negative results for each test. 3. Correctly perform specific gravity. 4. Correctly identify and enumerate microscopic examinations of urine specimens. 2/17/2016 8 5. Correctly perform sulfosalicytic acid precipitation test, the copper reduction test for sugar, and the Ictotest for bilirubin. 6. Correctly perform two CSF chamber counts and count WBC’s to within +/-10 of the instructor’s result. These activities precede student on-site affiliate experiences and are meant to prepare them to some degree for that experience. General Notes: Library assignments, periodicals, computerized modules, and guest speakers may be used as appropriate. Assessment of Student Learning: In both the didactic and the laboratory portions of the course, the student must achieve 78% or greater. Failure to achieve this minimum score will result in dismissal from the program. In the laboratory portion of the course, the final grade will be recorded as “Pass” or “Fail” and registered with the didactic portion. The following grading scale applies to all programs within the Allied Health Division: 100 – 92% = A 83 – 91% = B 78 – 82% = C 66 – 77% = D 65% and below = F In the lecture portion of the clinical course, the final grade is derived from student performance on examination(s) and/or assignments. Grading/Student Assessment of lecture (didactic) portion of the course: Final grade will be composed of: 1. Unit tests averaged = 60% 2. Case studies, study questions, or quizzes averaged = 10% 3. Final exam = 30% TOTAL 100% 2/17/2016 9 Grading/Student Assessment of laboratory (student laboratory and clinicals) portion of the course: 1. Skills Checklist: Passing is 78% of the total points available 2. Professional Behaviors Evaluation: Passing is no less than 22 points or 78% Final Pass/Fail clinical grade is the average of the Skills Checklist and the Professional Behaviors Evaluation Statement to Connect Course with General Education Outcomes or Technical Program Outcome Statement: In compliance with MACC’S General Education outcomes, the student who successfully completes this course will be able to: I. Demonstrate effective written and oral communication skills. II. Demonstrate an understanding of scientific principles and computational skills and how to use them to solve problems and make informed decisions. Program Outcomes and Assessments: The Allied Health Department continually strives to meet the needs of the Medical Laboratory Technician student through program improvements. This is a cooperative effort that includes input from the faculty, student, Medical Laboratory Technician Advisory Board, and other appropriate agencies or entities. Students are assessed on mastery of the course concepts and essential skills throughout the courses of the Medical Laboratory Technician Program. Other program assessments include clinical performance criteria, essential skills mastery, the clinical process evaluation, ASCP examination scores, placement rates, and follow-up surveys. Instructor Policies/Expectations: Instructors of this program expect the following from students: 1. Come to class prepared to discuss or apply important concepts by having read the assigned material or reviewed materials for instrument operation. 2. Participate in class by listening, taking notes, and making contributions to discussions. 3. Consult with faculty for clarification of difficult material or additional resources to consult. 4. Respect the learning environment by averting distractions and disturbances such as ringing cell phones and extraneous conversation in class. 5. Treat instructors and fellow students with consideration, concern, and fairness. Academic Dishonesty: MACC board policy is as follows: “Academic dishonesty by students damages institutional credibility and unfairly jeopardizes honest students; therefore, it will not be tolerated in any form.” Forms of academic dishonesty include but are not limited to the following: violations of copyright law, plagiarism, fabrication, 2/17/2016 10 cheating, collusion, and other academic misconduct. Incidents of dishonesty regarding assignments, examinations, classroom/laboratory activities, and/or the submission of misleading or false information to the College will be treated seriously. The procedure for handling academic dishonesty is outlined in the Student Handbook (Policy Handbook M.010). In cases of alleged academic dishonesty, the burden of proof is on the student, not on the instructor. Attendance: Students are expected to prepare for and attend all classes and clinical practice. Regular attendance improves probability for success in the program. Habitual tardiness and frequent absences are disruptive to the classroom and cause an unsafe environment in the student laboratory. Instructors carefully plan learning experiences, so it is important as a matter of courtesy and fairness to the class that all individuals be present. Students absent for reasons beyond their control, such as verified personal illness or family illness and/or death, can make up class work. If a student misses so many classes due to extenuating circumstances that the instructor feels the student cannot catch up, the MLT Program Coordinator will send a written report to the Director of Allied Health. Students who miss two consecutive weeks of class during a regular sixteen-week semester or the equivalent ratio of class time during a shorter session will be dropped from that class unless acceptable justification is supplied to the instructor and the Dean of Student Services. Additionally, students who miss more than one-fourth of the class meetings during any scheduled session may be dropped from that class by the instructor if, in the opinion of the instructor, the student does not have a reasonable opportunity to succeed in the class. Tardiness, make-up, and late work: Tardiness to class and clinicals is disruptive and inconsiderate of others. Being on time is mandatory. Make-up and late work: See the MLT Student Handbook for guidelines. Remember that communication, accountability and responsibility are very important professional behaviors. Refer to the MLT handbook for the following policies: Drop policy Drug/alcohol policy Grade appeal procedure Student code of conduct Student due process and grievance procedure Student rights and privacy act Use of computing resources ADA Statement Students who have disabilities that qualify under the Americans with Disabilities Act may register for assistance through the Office of Access 2/17/2016 11 and ADA Services. Students are invited to contact the Access Office to confidentially discuss disability information, academic accommodations, appropriate documentation and procedures. For more information, please call either the Moberly office at (660) 263-4100 x 11240 or the Columbia office at (573) 234-1067 x 12120, or visit our web page at http://www.macc.edu/index.php/services/access-office. 2/17/2016 12