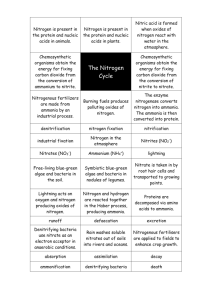
nitrogen terms
advertisement

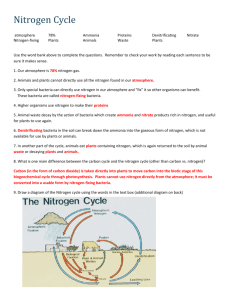
Nitrogen cycle a. Most abundant element in the atmosphere- 78% b. Cannot be absorbed or used( metabolized) directly as a nutrient by plants and animals c. Lightning- N2 + O2 -- 2NO d. Certain bacteria in the soil and aquatic systems convert nitrogen gas into compounds that can enter the food webs as a useful nutrient. e. Several major steps i. Nitrogen fixation 1. specialized bacteria convert N2-- NH3 that can be used in the reaction N2 + H2-- NH3 a. done by cyanobacteria in soil and water b. Rhizobium bacteria living in small nodules in the roots of some plants species ii. Nitrification1. 2 step process in which most of the ammonia in the soil is converted by specialized aerobic bacteria a. nitrite ions b. nitrate ions which are easily taken up by plants as a nutrient iii. Assimilation 1. plant roots absorb inorganic ammonia , ammonia ions and nitrate ions formed by nitrogen fixation and nitrification of soil water. 2. They use these ions to make nitrogen containing organic molecules such as DNA , amino acid s and proteins. Animals in turn get their nitrogen by eating plants and plant- eating animals iv. Ammonification 1. vast armies of specialized decomposer bacteria convert nitrogen rich organic compounds , wastes, cast off particles and dead bodies of organisms into a. simpler nitrogen containing inorganic compounds such as ammonia b. water soluble salts containing ammonia ions(NH4+) v. Denitrification 1. specialized bacteria( mostly anaerobic in water logged soil such as in an estuary, wetland, ocean , lake or river sediment, swamps, bogs) convert NH3 and NH4+ back to NO2-1, and NO3-1 and then in to N2 and N2O. These are then released back into the atmosphere to begin the cycle again. Nitrogen cycle f. Most abundant element in the atmosphere- 78% g. Cannot be absorbed or used( metabolized) directly as a nutrient by plants and animals h. Lightning- N2 + O2 -- 2NO i. Certain bacteria in the soil and aquatic systems convert nitrogen gas into compounds that can enter the food webs as a useful nutrient. j. Several major steps i. Nitrogen fixation 1. specialized bacteria convert N2-- NH3 that can be used in the reaction N2 + H2-- NH3 a. done by cyanobacteria in soil and water b. Rhizobium bacteria living in small nodules in the roots of some plants species ii. Nitrification1. 2 step process in which most of the ammonia in the soil is converted by specialized aerobic bacteria a. nitrite ions b. nitrate ions which are easily taken up by plants as a nutrient iii. Assimilation 1. plant roots absorb inorganic ammonia , ammonia ions and nitrate ions formed by nitrogen fixation and nitrification of soil water. 2. They use these ions to make nitrogen containing organic molecules such as DNA , amino acid s and proteins. Animals in turn get their nitrogen by eating plants and plant- eating animals iv. Ammonification 1. vast armies of specialized decomposer bacteria convert nitrogen rich organic compounds , wastes, cast off particles and dead bodies of organisms into a. simpler nitrogen containing inorganic compounds such as ammonia b. water soluble salts containing ammonia ions(NH4+) v. Denitrification 1. specialized bacteria( mostly anaerobic in water logged soil such as in an estuary, wetland, ocean , lake or river sediment, swamps, bogs) convert NH3 and NH4+ back to NO2-1, and NO3-1 and then in to N2 and N2O. These are then released back into the atmosphere to begin the cycle again.