Interlaboratory Method Comparison Stage 1
advertisement

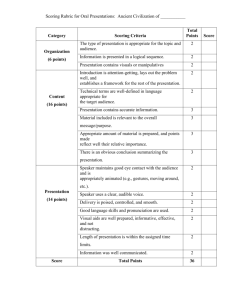
Interlaboratory Method Comparison was initiated by HUMN in 1999. A complete package of slides and instructions was sent to all participants in September 2000. The outline and information below are by Michael Fenech. This document's outline allows you to go directly to each chapter by clicking on the link. Objective/Aims Study design Instructions Scoring Procedure Scoring Criteria Photomicrographs All results were electronically transferred to Dr. Stefano Bonassi by February 2001. Your contribution is most appreciated. We hope you have found this exercise a positive experience. Two papers describing this international effort are in preparation. 1. Objective/Aims The objective of this project is to determine the extent of variability in MN frequency due to visual scoring of slides and staining method. The specific aims are: 1. To determine the extent of inter-laboratory variation in the micronucleus assay scores when a common set of scoring criteria are used to score cells sampled from the same culture. 2. To determine the extent of variation in the micronucleus assay scores obtained by the same scorer using the same slide. 3. To determine the effect of different staining methods on the micronucleus assay scores. 4. To compare the scores of participating laboratories to that of a single reference laboratory experienced in the criteria recommended for the study and in scoring the type of cell preparation used in the study. 2. Study design The study design is shown schematically in Figure 1. 100 ml BLOOD SAMPLE FROM YOUNG MALE ISOLATE LYMPHOCYTES (66x106 MINIMUM) Control 1Gy 2Gy 22x106 22x106 cells cells 22x106 cells 22ml 22ml 22ml STANDARD CYTOKINESIS-BLOCK MICRONUCLEUS ASSAY prepare slides using DMSO method spot a spot a spot b spot b slides fixed and stained slides fixed only slides fixed and stained slides fixed only slides fixed and stained slides fixed only code slides code slides code slides MF scores slides MF scores slides MF scores slides to each lab send set of instructions one set of fixed and stained slides (con, 1Gy, 2Gy) one set of fixed only slides (con, 1Gy, 2Gy) scoring procedure with diagrams & photos Excel template for recording scores data to Stefano Bonassi A blood sample was collected from a healthy 30 year-old male. Lymphocytes were isolated and three cultures in RPMI 1640 with foetal calf serum were prepared. Two of the cultures were exposed to gamma-rays (1Gy or 2Gy at a dose rate of 5Gy/minute). The lymphocytes were stimulated to divide with PHA and 44h later cytochalasin-B (4.5ug/ml) was added and the cells harvested 28h later. A cytocentrifuge was used to prepare slides with 2 spots of cells on each slide. The cells were air-dried and then fixed in absolute methanol. Half of the slides were stained with Diff-Quik and the rest left unstained and stored desiccated at 4OC. The stained slides were mounted in DEPEX using a coverslip. Forty-seven stained and unstained slides were prepared from each culture. The slides were coded using random numbers and a sub-sample of the slides (6 from each culture) were scored by one person at the reference laboratory prior to sending the slides. Each participating laboratory is expected to obtain separate scores from each spot on each slide, thus giving duplicate measurements for each slide from which a coefficient of variation can be estimated. Each laboratory may choose to have more than one person scoring the same set of slides provided but a separate data sheet has to be generated for each scorer. 3. Instructions 1. Unpack carefully the boxes in which the slides have been packed. 2. If any of the slides are broken please notify us (julie.turner@hsn.csiro.au) immediately so we can send you replacement slides made from the same culture. 3. Stain the unstained slides immediately when you receive them. Otherwise keep them desiccated at 4oC until you stain them. 4. Read the scoring procedure and scoring criteria carefully. Do not proceed with scoring if you are uncertain about any aspect of the scoring procedure. Please seek clarification from us (michael.fenech@hsn.csiro.au) before proceeding with scoring. 5. Record the data in the Excel template provided. Send the excel template with your results to Dr. Stefano Bonassi by email bonassi@hp380.ist.unige.it . 6. Complete scoring, send data to Stefano by ***December 30, 2000*** 4. Scoring Procedure 1. Each scorer in your laboratory is to score the full set of slides (pre-stained and those stained in your laboratory; total of six slides). Each scorer is to obtain separate scores for the cells in the spot closest to the label (spot a) and the cells in the spot farthest from the label (spot b); (total of twelve spots). Preferably cells in spot a and spot b should be scored on separate days. This will enable us to take into account the day to day variation in scoring. 2. For each spot of cells score the following cells to determine the nuclear division index: number of mononucleated (MONO) cells in 500 viable cells number of binucleated (BN) cells in 500 viable cells number of multinucleated (MULT) cells in 500 viable cells It is important to note that you are not to score necrotic or apoptotic cells when determining the proportion of MONO, BN and MULT cells. Please also note that you are not required to distinguish between tri-nucleated and quadri-nucleated cells when scoring multinucleated cells because often it is hard to distinguish between the two and this adds an unnecessary burden to the assay. 3. For each spot of cells score the following to determine chromosome damage: the number of BN cells containing one or more micronuclei in 1000 BN cells the total number of micronuclei in 1000 BN cells the number of BN cells containing one or more nucleoplasmic bridges (NPBs) in 1000 BN cells In the case of the latter please record a BN cell that has both a micronucleus and an NPB separately as a BN cell with micronucleus and separately as a BN cell with a NPB. To simplify the project, you are not requested to determine the distribution of micronuclei in BN cells. This can be inferred from the ratio of the number of MNi in 1000 BN cells and the number of BN cells with MNi in 1000 BN cells. Examine all slides at 1000 times magnification for both light or fluorescence microscopy Do not score cells which you are unable to classify according to the criteria described below. Simply skip the cell and move on to the next. Similarly, only score micronuclei and nucleoplasmic bridges when you are confident that they meet the criteria described below. 5. Scoring Criteria Criteria for selecting binucleated cells which can be scored for micronucleus frequency The cytokinesis-blocked cells that may be scored for MN frequency should have the following characteristics: The cells should be binucleated; The two nuclei in a binucleated cell should have intact nuclear membranes and be situated within the same cytoplasmic boundary; The two nuclei in a binucleated cell should be approximately equal in size, staining pattern and staining intensity; The two nuclei within a BN cell may be attached by a fine nucleoplasmic bridge which is no wider than 1/4th of the largest nuclear diameter. The two main nuclei in a BN cell may touch but ideally should not overlap each other. A cell with two overlapping nuclei can be scored only if the nuclear boundaries of each nucleus are distinguishable. The cytoplasmic boundary or membrane of a binucleated cell should be intact and clearly distinguishable from the cytoplasmic boundary of adjacent cells. Examples of the type of binucleated cells that may or may not be scored are illustrated diagrammatically in figure 2 and figure 7. The cell types that should not be scored for micronucleus frequency include mono-, tri-, quadr- and multi-nucleated cells, and cells that are necrotic or apoptotic (illustrated in figures 3 and 7). Criteria for scoring micronuclei (MNi) MNi are morphologically identical to but smaller than nuclei. They also have the following characteristics The diameter of MNi in human lymphocytes usually varies between 1/16th and 1/3rd of the mean diameter of the main nuclei which corresponds to 1/256th and 1/9th of the area of one of the main nuclei in a BN cell, respectively. MNi are non-refractile and they can therefore be readily distinguished from artefact such as staining particles; MNi are not linked or connected to the main nuclei ; MNi may touch but not overlap the main nuclei and the micronuclear boundary should be distinguishable from the nuclear boundary; MNi usually have the same staining intensity as the main nuclei but occasionally staining may be more intense. Examples of typical MNi that meet the criteria set above are shown in figures 4 and 7. Examples of cellular structures that resemble MNi but should not be classified as MNi originating from chromosome breakage or loss are illustrated in figures 5 and 7. Criteria for scoring nucleoplasmic bridges Nucleoplasmic bridges are sometimes observed in binucleated cells following exposure to clastogens. These bridges are a continuous link between the nuclei in a binucleated cell and are thought to be due to dicentric chromosomes in which the centromeres were pulled to opposite poles during anaphase. The width of a nucleoplasmic bridge may vary considerably but usually does not exceed 1/4th of the diameter of the nuclei within the cell. The nucleoplasmic bridge should have the same staining characteristics of the main nuclei. On very rare occasions more than one nucleoplasmic bridge may be observed within one binucleated cell. A binucleated cell with a nucleoplasmic bridge often contains one or more micronuclei. Examples of binucleated cells with nucleoplasmic bridges are illustrated in figure 1,4 and 7. Criteria for scoring apoptotic and necrotic cells Apoptotic cells: cells with chromatin condensation and intact cytoplasmic and nuclear boundaries (“early” apoptotic cells) or cells exhibiting nuclear fragmentation into smaller nuclear bodies within an intact cytoplasm/cytoplasmic membrane (“late” apoptotic cells). Necrotic cells: cells exhibiting a pale cytoplasm with numerous vacuoles (mainly in the cytoplasm and some in the nucleus) and damaged cytoplasmic membrane with a fairly intact nucleus (“early” necrotic cells) or cells exhibiting loss of cytoplasm and damaged/irregular nuclear membrane with only a partially intact nuclear structure (“late necrotic cells). Figures 3, 6 and 7 illustrate typical examples of necrotic and apoptotic cells. Binucleated cells which can be scored for MNi Figure 2. Criteria for choosing binucleate cells in the cytokinesis-block micronucleus assay. A. ideal binucleate cell; B. Binucleate cell with touching nuclei; C. Binucleate cell with narrow nucleoplasmic bridge between nuclei; D. Binucleate cell with relatively wide nucleoplasmic bridge. Cells with two overlapping nuclei may be considered suitable to score as binucleated cells if the nuclear boundaries are distinguishable. Occasionally binucleated cells with more than one nucleoplasmic bridge are observed. [A] mono- and multinucleate cells [B] apoptotic cells [C] necrotic cells Figure 3. The various types of cells that may be observed in the in vitro cytokinesis-block micronucleus assay excluding binucleated cells. These cell types shown should not be scored for MN frequency : [A] viable mono-, tri- and quadrinuclear cells; [B] mono- and binucleated cells at early stage of apoptosis when chromatin condensation has occurred but nuclear membrane has not disintegrated and late stage apoptotic cells with intact cytoplasm, no nucleus and apoptotic chromatin bodies within the cytoplasm; [C] cells at the various stages of necrosis including early stages showing vacuolisation, disintegration of cytoplasmic membrane and loss of cytoplasm with an intact nucleus and late stages in which cytoplasm is partially or completely lost and nuclear membrane is visibly damaged and nuclear material is commencing to leak from the remnant nucleus. Micronucleus that meet the scoring criteria Figure 4. Typical appearance and relative size of micronuclei in binucleated cells. [A] Cell with two micronuclei one with 1/3rd and the other 1/9th the diameter of one of the main nuclei within the cell. [B] Micronuclei touching but not overlapping the main nuclei. [C] A binucleated cell with nucleoplasmic bridge between main nuclei and two micronuclei. [D] A binucleated cell with six micronuclei of various sizes; this type of cell is rarely seen. Cellular structures that resemble MNi but should not be scored as MNi Figure 5. Occasionally binucleated cells (or cells that resemble binucleated cells) may contain structures that resemble micronuclei but should not be scored as micronuclei originating from chromosome loss or chromosome breakage. These situations include [A] A trinucleated cell in which one of the nuclei is relatively small but has a diameter greater than 1/3 the diameter of the other nuclei; [B] dense stippling in a specific region of the cytoplasm ; [C] extruded nuclear material that appears like a micronucleus with a narrow nucleoplasmic connection to the main nucleus and [D] nuclear blebs that have an obvious nucleoplasmic connection with the main nucleus. Figure 6. The various possible fates of cultured cytokinesis-blocked cells following exposure to cytotoxic/genotoxic agents. 6. Photomicrographs The following are photomicrographs of typical cells in the cytokinesis-block micronucleus assay with cultured lymphocytes [I] Mononucleated (MONO) cells. Varying sizes of lymphocytes are shown with increasing size and increasing cytoplasmic volume/nuclear volume ratios. (d) and (e) also exhibit nuclear extrusions. [a] [d] [b] [c] [e] [f] MONO cells [II] Multinucleated [MULT] cells. The number of obvious nuclei may vary between three and eight nuclei depending on the number of nuclear divisions occurring since cyt-B addition. Sometimes it is difficult to determine the precise number of nuclei due to overlap and connections between nuclei. [a] [b] [d] MULT cells [III] Typical binucleated (BN) cells. (d) and (e) illustrate cells in which the nuclei have an indentation; these are occasionally seen and should be considered as normal binucleated cells as they are not related to genotoxic exposure. [a] [d] [b] [c] [e] [f] BN Cells without MN [IV] Binucleated cells (BN) with micronuclei (MN). (d) illustrates a micronucleus touching both nuclei. [a] [d] [b] [c] [e] [f] BN Cells with MN [V] Binucleated (BN) cells with both micronuclei (MN) and nucleoplasmic bridges (NPB). (c) and (d) show a BN cell with two NPB. (e) shows a BN cell with a very short NPB. [a] [b] [c] [d] [e] [f] BN Cells with MN and NPB [VI] Binucleated (BN) cells with nucleoplasmic bridges (NPB) but no micronuclei (MN). (e) illustrates a relatively wide nucleoplasmic bridge. [a] [d] [b] [c] [e] [f] BN Cells with NPB only [VII] Structures that resemble micronuclei (MNi) but should not be classified as MNi. (a) a BN cell with a micronucleus (on the right) and two nuclear blebs (on the left) indicated by an arrow; the lower bleb is surrounded by a vacuole. (b) – (d) BN cells with nuclear blebs. (e) and (f) Trinucleated cells with one small nucleus which resembles a micronucleus but has a diameter greater than 1/3 the large diameter of the two main nuclei. [a] [b] [c] [d] [e] [f] BN cells with non-MN structures [VIII] Necrotic cells. (a) – (c) cells in the early stages of necrosis when the cytoplasm is vacuolated and pale and the nucleus is only marginally intact. (d) - (g) cells in the late stages of necrosis when the cytoplasm and cytoplasmic membrane are lost and the nucleus is clearly not intact and in a state of degeneration. [a] [e] [b] [c] [f] [g] [d] Necrotic Cells [IX] Apoptotic cells. (a) – (c) mononucleated cells at various stages of apoptosis showing condensed chromatin in the nucleus and a dark cytoplasmic staining. (d) and (e) a BN cell undergoing apoptosis. (f) a cell at the very late stage of apoptosis when the nuclear material has completely broken down within an intact cytoplasm and cytoplasmic membrane. An unusual feature about (d) is the presence of large vacuoles in the cytoplasm. [a] [b] [c] [d] [e] [f] Apoptotic Cells Figure 7. Microphotographs.