Fabrication of Co3O4 nanowall and nanowire via thermal anneal on
advertisement
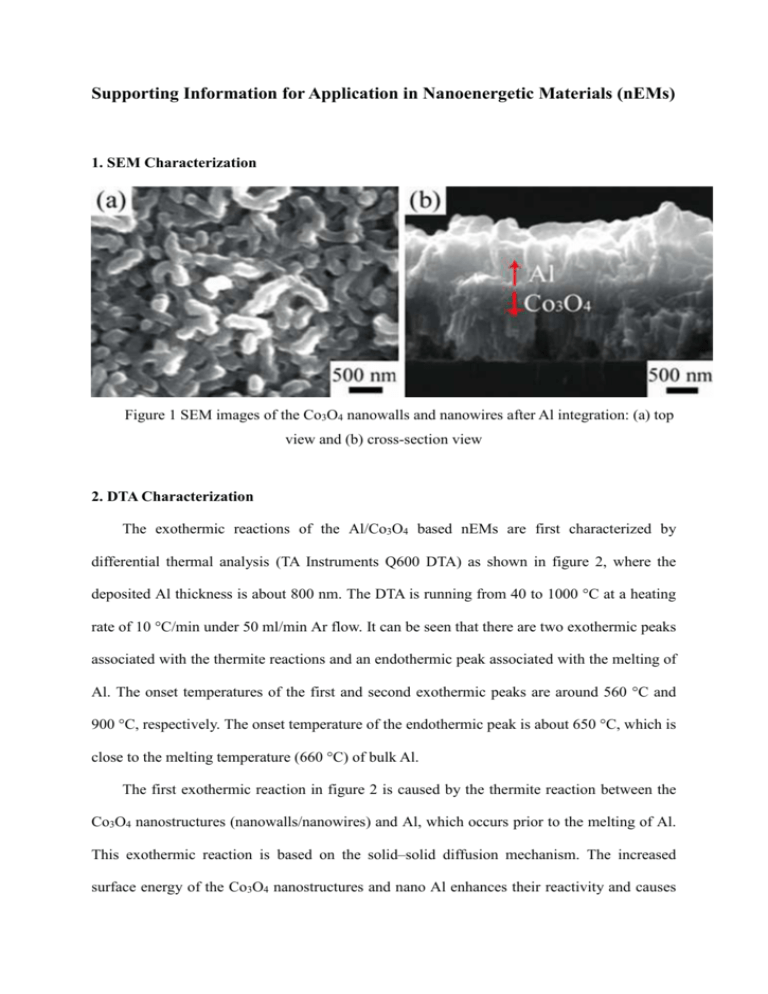
Supporting Information for Application in Nanoenergetic Materials (nEMs) 1. SEM Characterization Figure 1 SEM images of the Co3O4 nanowalls and nanowires after Al integration: (a) top view and (b) cross-section view 2. DTA Characterization The exothermic reactions of the Al/Co3O4 based nEMs are first characterized by differential thermal analysis (TA Instruments Q600 DTA) as shown in figure 2, where the deposited Al thickness is about 800 nm. The DTA is running from 40 to 1000 °C at a heating rate of 10 °C/min under 50 ml/min Ar flow. It can be seen that there are two exothermic peaks associated with the thermite reactions and an endothermic peak associated with the melting of Al. The onset temperatures of the first and second exothermic peaks are around 560 °C and 900 °C, respectively. The onset temperature of the endothermic peak is about 650 °C, which is close to the melting temperature (660 °C) of bulk Al. The first exothermic reaction in figure 2 is caused by the thermite reaction between the Co3O4 nanostructures (nanowalls/nanowires) and Al, which occurs prior to the melting of Al. This exothermic reaction is based on the solid–solid diffusion mechanism. The increased surface energy of the Co3O4 nanostructures and nano Al enhances their reactivity and causes the reduced onset temperature. After the melting of Al, Co3O4 and tiny CoO beneath the Co3O4 nanostructures react with the melted Al at about 900 °C. The second exothermic reaction is based on the liquid–solid diffusion mechanism. Figure 2 DTA curve of the reactions of the Al/Co3O4 based nEMs 3. DSC Characterization In order to quantitatively determine the heat of reaction, the Al/Co3O4 based nEMs are characterized using differential scanning calorimetry analysis (TA Instruments Q20 DSC) as shown in figure 3. The DSC is carried out from 40 to 680 °C at a heating rate of 5 °C/min under 75 ml/min Ar flow. The exothermic peak with a peak temperature of about 560 °C corresponds to the first exothermic peak in the DTA curve in figure 2, which is caused by the solid–solid reaction between the Co3O4 nanostructures and the nano Al. Integration of this exothermic peak in the DSC curve gives a heat of reaction of 1770 J/g. The endothermic peak with a peak temperature of about 660 °C corresponds to the endothermic peak in the DTA curve in figure 2, which is caused by the melting of Al. By assuming that the DTA signal is proportional to the heat of reaction, we can use the value of heat of reaction calculated from the DSC analysis in figure 3 to scale the DTA curve in figure 2. Although this is not very accurate, because the relationship between the temperature and the heat is not exactly linear, it allows us to roughly estimate the total heat of reaction of the Al/Co3O4 based nEMs to be around 3100 J/g. Figure 3 DSC curve of the reactions of the Al/Co3O4 based nEMs











