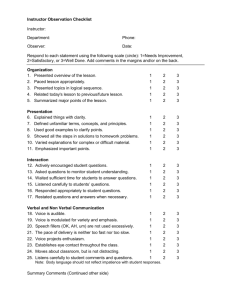
CHM 121 Prep Sheet – Experiment 2 Paper Chromatography
advertisement

Last modified in January 2010 by Gary Mines Instructor Notes for CHM 122 Experiments Exp 7. Analysis of an Unknown Chloride (Slowinski, 8th Ed.) Instructor Notes The %Cl's for each of the unknowns is: 14.27%, 19.02%, and 23.78% (NOTE: It is difficult to get these unknowns (pseudo) homogeneous since they are mixtures of two different salts. Thus it has not been uncommon for reproducibility and accuracy to be somewhat poor for reasons other than technique. We tried a new technique of “homogenization” using a coffee grinder in Fall ’09, but I did not solicit feedback from instructors. We will do this again in S’10 and I will gather data in my classes to see if this solves the problem. Please feel free to provide me data from your classes or personal feedback about this issue. Exp. 20. Rates of Chemical Reactions, I. The Iodination of Acetone (Parts A, B, and C) (Slowinski, 8th Ed.) Instructor Notes The reaction is 1st order in both acetone and H+ (and zero order in I2, as stated in manual) In Fall '06, the times for trial 1 in my classes were about 300 +/- 20 seconds and k was about 2.2 (+/- 0.2) x 10-5 M-1s-1. Oddly enough, in Spring '07, the times for trial 1 were significantly different—about 150 +/- 20 s and k was about 4.4 (+/- 0.6) x 10-5 M-1s-1. The lab manual authors give a value of 2.7 x 10-5 M1 -1 s in their sample data set. Exp. 21. Rates of Chemical Reactions, II. A Clock Reaction (Parts A and B) (Slowinski, 8th Ed.) Instructor Notes Rxn is 1st order in I- and BrO3-; 2nd order in H+. Ea (lit) = 41.4 kJ/mol (values from 35-50 kJ/mol are often observed in my classes when data are properly worked up [and S2O32- is fresh]). The initial trial can sometimes take longer than 90 seconds [I've seen 200 s!], despite what the lab manual says (if it is significantly less than 90 seconds, speak with the lab paraprofessional about getting new Na2S2O3(aq). The 0 degree trial can often take between 7-10 minutes, so to save them time, I usually ask them to start that reaction before doing the high temperature mixture so that they can be preparing the high temperature trial while they're waiting for the zero degree trial to finish. I have found the 10-degree trial to be problematic (probably due to inaccurate/unstable T) and so I have omitted that trial completely. Last modified in January 2010 by Gary Mines Exp 23. Determination of the Equilibrium Constant for a Chemical Reaction (Equation) (Slowinski, 8th Ed.) Instructor Notes If you'd like an electronic version of the calibration curve, you can download it from http://www.oakton.edu/~gmines/adjuncts/122/ NOTE: Starting in S’10, Spec Genesys instruments will be used at both RHC and DP so there will be only one calibration curve (for both campuses). Exp 36. Qualitative Analysis of Group I Cations (Slowinski, 8th Ed.) Exp. 26. Determination of the Solubility Product of PbI 2 (Slowinski, 8th Ed.--NOTE: This experiment has been changed in the 9th Ed. of this manual but we will not be doing that changed version) Instructor Notes Change in procedure comment: Students will NOT follow the instructions exactly as stated in the lab manual (for the oxidation of iodide procedure), since our cuvette tubes are not ideally suited for that. Instead, students will use special provided test tubes (rather than cuvettes) as the vessels in which to do the final oxidation of iodide by nitrite. These tubes have been pre-scored/marked in the same manner as the manual indicates for (their) cuvettes. Student groups will each get 5 of these test tubes dry from a rack provided. Once the appropriate additions are made to each tube (and mixed), students will pour a portion of the contents into a disposable square cuvette (at DP) or a round glass cuvette (at RHC, for now) to make the absorbance measurement. IT IS IMPORTANT THAT STUDENTS RINSE THEIR SET OF THE PRE-SCORED TUBES WITH DEIONIZED WATER AND RETURN THEM TO THE RACK PROVIDED SO THAT THEY CAN BE DRIED FOR THE NEXT CLASS’S USE. Test tube clarification: Tell students that where the lab manual says “regular” test tube, they should use a “large” test tube (i.e., the largest ones in their drawer). When the manual says “small” test tube, they should use a “medium” test tube (the one they have twelve of in their drawer [they only have six of the largest and smallest tt’s]). Helpful advice to students: It will make students’ lives considerably easier down the line if they 1) really let tubes 1-4 settle well (at least the 3-4 minutes stated in the manual) and 2) are careful when decanting the supernatant into their medium test tube the first time (and every time thereafter, I suppose). If students are sloppy in their transfers, they will not get rid of all the yellow crystals, and even a tiny amount of these can cause substantially large absorbance errors (on the high side) once the KNO2 (oxidizing agent) is added. I suspect that if a group comes to you saying that their value of absorbance is “off the scale” of the calibration plot, they probably had extra yellow crystals at the time of oxidation. “Big Picture” Advice: Students will have a difficult time seeing the “big picture” for this experiment. The write up is short, but confusing because a) tubes 1-4 are treated differently than tube 5 [for a challenging prelab question, ask them why—only the best students will be able to answer this, and only typically after some thought!], b) Last modified in January 2010 by Gary Mines many of the steps seem similar, and c) the details really matter here, even more than “usual” [students can easily discard the wrong “part” after centrifuging]. I have had better success recently by doing a kind of flow chart (with sketches of test tubes) on the board during prelab and leaving it up on the board during the lab to help them. If you’d like to see roughly what I do, let me know. Separately, I have created an alternative report form for this experiment that has students set up “ICE” type tables in an attempt to “connect” this lab to many of their homework equilibrium problems, which (in my case) are done generally in terms of concentrations rather than moles. Let me know if you’d like to see this form. If you'd like an electronic version of the calibration curve, you can download it from http://www.oakton.edu/~gmines/adjuncts/122/ NOTE: Starting in S’10, Spec Genesys instruments will be used at both RHC and DP so there will be only one calibration curve (for both campuses). Exp 25. pH Measurements—Buffers and Their Properties (B and C) (Slowinski, 8th Ed.) Instructor Notes I have changed the prep sheet so that your class can now have the three choices of buffer system mentioned in the lab manual for Part C (I had previously had it so that only the acetic acid/acetate buffer system was to be used, although I failed to write this up in the instructor notes. My apologies for any past inconvenience/confusion this may have caused.) Note: If anyone feels that they’d like to add Part A and/or D in future semesters (or have chemicals available for a demo of, say, Part A [indicators]), please let me know. As it currently stands, the materials provided to your class will be for Parts B and C only. Acid-Base Titration Curve Instructor Notes pKa (mandelic acid) = 3.41 (Bjerrum, J. 1958) pKa (glycolic acid) = 3.83 pKa (NH4+) = 9.25 (Calculated using Kb(NH3) = 1.76 x 10-5; textbook) Since the pKa for NH4+ ion is high (9.25) [i.e., NH4+ is a pretty weak weak acid], there is no "sudden rise" at the equivalence point. An inflection point can be seen, but it is obviously less clear-cut than in the stronger acid cases. If you wish to avoid this confusion (and the confusion that there is a sharp rise in pH at the beginning of the titration—many students feel that something "must be wrong" at this point), you may simply tell students not to choose the NH4Cl. OR....The experiment itself (not the explanation/theory/calculations part) is fairly short, so one adjunct instructor has suggested having students do either glycolic or mandelic first and then do a second titration using NH4Cl. This way the effect of pKa on the shape of the titration curve can be more directly witnessed/experienced by students. I have not tried this (I tend to spend a lot of time on theory during prelab) but you may feel free to do so, as long as you tell me ahead of time so that extra standardized NaOH(aq) can be prepared for your lab period. Last modified in January 2010 by Gary Mines Exp. 38. Qualitative Analysis of Group III Cations Exp. 32. Voltaic Cell Measurements Instructor Notes A slightly different apparatus is used (the outer beaker is replaced by a plastic “cup”; the inner crucible with frit is replaced with a narrow ceramic (porous material) “cup”. Because of this different apparatus, you need to tell students to add 20 mL (not 10 mL, as in the procedure) of solution to the outside cup, and 10 mL to the inner cup. SINCE THE OUTSIDE CUP IS SO LARGE, YOU MUST MAKE SURE THAT YOUR STUDENTS USE A GRADUATED CYLINDER SO THAT THEY DON'T USE MORE THAN 20 mL. If students use more than 20 mL, you will likely run out of reagent allotted for your section. A more detailed document discussing the new apparatus will be sent via email to instructors upon request. Exp. 31. Determination of an Equivalent Mass by Electrolysis Instructor Notes Care must be taken not to have the clip holding the anode contact the solution in the beaker, particularly since most students don't recognize that as the electrolysis takes place, the liquid level in the beaker is constantly rising (because as hydrogen gas is produced it displaces liquid in the buret into the beaker). Students may need to adjust the position of the clip during the electrolysis! Exp. 33 Preparation of Copper(I) Chloride (Optional Part NOT included) Exp. 28. Determination of the Hardness of Water Change in procedure: NOTE: I have halved the [EDTA] from that in the instructor manual in order to get reasonable volumes of EDTA(aq) per titration with 50 mL of water sample for titration. This also requires that students use about half as much CaCO3(s) as stated in the lab manual procedure (see below). Instructor Notes 1) YOU MUST TELL STUDENTS TO TAKE NO MORE THAN ~0.16 g OF CaCO3(s) (RATHER THAN 0.4 g) TO PREPARE THEIR STANDARD SOLUTION. (See note in prior paragraph.) 2) Although we provide 25.00-mL and 50.00-mL pipets, I give students the option to use a buret in place of the pipets to deliver "known volumes" of their standard carbonate solution and water samples to the Erlenmeyer flasks for titration. Some adjuncts have reported using a graduated cylinder for this, but to me that would send a poor message about accuracy and precision. On the other hand, the Last modified in January 2010 by Gary Mines endpoint in this titration is “slow” to develop and harder to see, generally, so the precision is typically not that great anyway. 3) An alternate report form I created is available if you wish at www.oakton.edu/~gmines/Adjuncts/122. I also wrote a short handout to help clarify some features of this experiment that you are free to use if you wish (found at same place on web). Exp. 41. Synthesis of Aspirin If you'd like an electronic version of the calibration curve for the salicylic acid impurity part of the experiment (although I consider this an optional part, which I have never actually done with my students), you can download it from http://www.oakton.edu/~gmines/adjuncts/122/ NOTE: Starting in S’10, Spec Genesys instruments will be used at both RHC and DP so there will be only one calibration curve (for both campuses).