Methemoglobinemia
advertisement

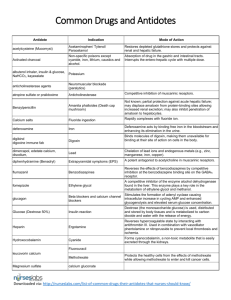
METHEMOGLOBINEMIA Mita Sanghavi Goel, M.D. March 13, 2003 Definition Methemoglobin is formed by oxidizing normal ferrous (Fe2+) hemoglobin to ferric (Fe3+) hemoglobin, which is not capable of binding oxygen or carbon dioxide. Clinical Presentation Depends on the level of methemoglobin 1.5-3.0 g/dL (10-20%) 3.0-4.5 g/dL (20-30%) 4.5-7.5 g/dL (30-50%) 7.5-10.5 g/dL (50-70%) >10.5 g/dL (>70%) cyanosis anxiety, lightheadedness, headache, tachycardia fatigue, confusion, dizziness, tachypnea, increased tachycardia coma, seizures, arrhythmia, acidosis death Pathophysiology Methemoglobin is regularly formed by oxidative stresses from a variety of sources, including oxygen free radicals. RBCs do not have the ability to synthesize new proteins and do not have mitochondria that generate energy to assist with redox reactions; therefore, RBCs (especially older ones) are highly susceptible to oxidation. About 1% of total hemoglobin exists as methemoglobin. Methemoglobin causes increased oxygen affinity in the remaining normal heme moieties and a leftward shift of the oxyhemoglobin dissociation curve. This leads to decreased release of oxygen and worsening tissue hypoxemia. Mechanisms of Reducing Methemoglobin Enzymatic Systems Nicotine Adenine Dinucleotide (NADH)-dependent Cytochrome b5 Methemoglobin Reductase o The major system for reduction of methemoglobinemia. Accounts for 99% of daily reduction of methemoglobin. o Can convert methemoglobin to hemoglobin at a rate of about 15% per hour. o Patients deficient in cytochrome b5 reductase can still maintain methemoglobin levels below 50%, which suggests that other minor pathways may become more active. Nicotine Adenine Dinucleotide Phosphate (NADPH)dependent Methemoglobin Reductase o Under normal conditions, this pathway has a minimal role in methemoglobin reduction o This pathway reduces methylene blue to leukomethylene blue, which in turn reduces methemoglobin to hemoglobin. Cellular Antioxidants Vitamin C, Glutathione, etc. o Normally play a small role in reducing methemoglobin, but may be upregulated in patients with NADH-dependent cytochrome b5 reductase deficiency. Causes Toxins In adults, the most common cause of methemoglobinemia is toxins. The most frequent offenders are benzocaine, nitrates, nitrites, anilines, naphthalene, and phenazopyridine (pyridium). Of note, dapsone and anilines produce a rebound methemoglobinemia 4-12 hours after treatment. Beth Israel Deaconess Medical Center Interns’ Report Genetic Generally present early in life. May be deficiencies in the enzymes for methemoglobin reduction, such as cytochrome b5 reductase. Also may be secondary to abnormal hemoglobin production of Hemoglobin M (autosomal dominant, heterogeneous). Diagnosis Pulse Oximetry Regular pulse oximetry relies on measuring the absorbance of 2 wavelengths of light, 660nm and 940 nm. Oxyhemoglobin and deoxyhemoglobin each absorb both wavelengths of light in different ratios. A ratio of 0.43 (660nm/940nm)=100% oxygen saturation and a ratio of 3.4=0% saturation. Methemoglobin also absorbs both wavelengths of light. 100% methemoglobin absorbs a ratio of 1 (660nm/940nm) and will register as 85% oxygen saturation. As a result, the lowest oxygen sat of 82-85% occurs at the absorbance ratio of 30-35% methemoglobin, unless deoxyhemoglobin is also present. The appearance of cyanosis despite a reasonable oxygen saturation should trigger the thought of methemoglobin. ABG Because it measures PO2 based on the oxygen dissolved in the blood rather than the oxygen bound to hemoglobin, PO2 will remain normal. Additionally, because the oxygen saturation is calculated from the pH and PCO2, assuming normal oxygenhemoglobin saturation curve and normal hemoglobin, the PO2 may appear normal. Cooximetry THE method of diagnosing methemoglobinemia, cooximetry must be separately ordered. Uses 4 wavelengths of light, which can distinguish between deoxyhemoglobin, oxyhemoglobin, carboxyhemoglobin, and hemoglobin. With the additional resolution from the wavelengths, cooximetry can measure the percentage of methemoglobin in a blood gas sample. Bedside Tests Methemoglobin has a characteristic chocolate-brown appearance that does not redden even when exposed to oxygen (unlike deoxygenated blood) Treatment Treatment is generally reserved for patients with a level of 20% in symptomatic patients and 30% in asymptomatic patients. Additional factors that may influence treatment are coexisting illnesses such as heart disease, lung disease, anemia, etc. Oxygen Although this does not assist with reducing methemoglobin, oxygen will decrease any effects of deoxyhemoglobin. Methylene blue The cornerstone of therapy. Methylene blue is reduced to leukomethylene blue by NADPH methemoglobin reductase, and then in turn, reduces methemoglobin to hemoglobin. Significantly reduced methemoglobin within one hour if it works. Dosed at 1-2 mg/kg (0.2mL/kg of a 1% solution) over 3-5 minutes and repeated after 30 minutes. CAUTIONS: o In patients with G6PD deficiency, methylene blue may produce a Heinz body hemolytic anemia. o Large doses (>4 mg/kg) may worsen methemoglobinemia because of its oxidizing property. o Is not effective in patients with NPDHP methemoglobin reductase deficiency. o Is not effective with sulfhemoglobinemia, which can be created when methemoglobin forms a covalent bond with sulfur. Dextrose Dextrose is necessary to form NADPH for the methylene blue to be effective. Miscellaneous Treatments Charcoal if necessary to reduce the enterohepatic circulation of some toxins, such as dapsone. P-450 inhibitors such as cimetidine and ketoconazole to block the metabolism of some toxins to their oxidizing forms. N-acetylcysteine (NAC) may be a treatment option for patients with G6PD deficiency. Hyperbaric oxygen and exchange transfusions may be necessary in some patients. Beth Israel Deaconess Medical Center Interns’ Report