Bioavailability, pharmacokinetics, and pharmacodynamics of
advertisement

Bioavailability, pharmacokinetics, and
pharmacodynamics of torsemide in patients
with cirrhosis
The bioavailability, pharmacokinetics, and pharmacodynamics of torsemide (10 mg orally and intrave
nously) were determined in a randomized crossover clinical trial with 12 patients with ascites caused by
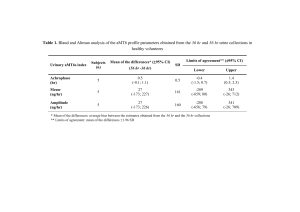
cirrhosis. Torsemide was rapidly absorbed with a bioavailability of 96.3% (confidence interval, 84% to
109%). Compared with healthy subjects, patients with cirrhosis exhibit a decrease in nonrenal clearance
and increases in bioavailability, volume of distribution, renal clearance, elimination half-life, and percent
age of the dose excreted into the urine. A greater proportion of the dose is delivered to the site of action
over a more prolonged period of time. In spite of a shift of the pharmacodynamic curve to the right in
patients with cirrhosis, there was no significant difference in natriuresis. Pharmacokinetic changes of
torsemide in cirrhosis therefore compensate for the pharmacodynamic abnormality. (CLIN PHARMACOL
THER 1993;54:90-7.)
Steven Schwartz, MD, D. Craig Brater, MD, David Pound, MD, Paula
K. Green, RN, William G. Kramer, PhD, and David Rudy, MD
Indianapolis, Ind., and Rockville, Md.
Torsemide
(torasemide)
(l-isopropyl-3-{[4-(3methyl-phenyl-amino)pyridine]-3-sulfonyl}urea) is a
diuretic of the pyridine sulfonylurea class undergoing
investigation for the treatment of edema caused by
congestive heart failure, renal insufficiency, and cirrhosis and for the treatment of hypertension. Ascites
and edema are frequent complications of cirrhosis.
Primary therapy consists of sodium restriction and
usually the administration of spironolactone, but loop
diuretics are often used for patients in whom this regimen fails. For rational dosing one should understand
the effects of hepatic disease on pharmacokinetics. In
addition, patients with cirrhosis usually exhibit di-
From the Divisions of Clinical Pharmacology and Gastroenterology,
Department of Medicine, Indianapolis, and Boehringer Mannheim Pharmaceuticals, Inc., Rockville.
Supported by a grant from Boehringer Mannheim Pharmaceuticals,
Inc. (Rockville, Md.). The General Clinical Research Center at
Indiana University School of Medicine is supported by grant
MORR00750-21 from the National Institutes of Health (Bethesda,
Md.).
Received for publication Jan. 11, 1993; accepted March 19, 1993.
Reprint requests: David Rudy, MD, Wishard Memorial Hospital,
OPW Bldg., Room 320, 1001 West Tenth St., Indianapolis, IN
46202-2879.
Copyright © 1993 by Mosby-Year Book, Inc.
0009-9236/93/$l.OO + 0.10 13/1/47266
90
uretic resistance because of a pharmacodynamic abnormality, the mechanism of which is unknown,
wherein there is a diminished response relative to the
amount of diuretic reaching the urine.1 The extent to
which pharmacodynamics are altered in patients with
cirrhosis should also be defined to allow better design
of diuretic regimens. Thus ample rationale exists for
assessing the pharmacokinetics and pharmacodynamics of torsemide in patients with cirrhosis.
In addition, disposition properties of torsemide allow its use to test a more general hypothesis.
Torsemide differs from other loop diuretics in that
about 20% of an intravenous dose reaches the urine as
unchanged drug; 80% of elimination occurs by hepatic
metabolism.2-6 In contrast, for furosemide and bumetanide, about half of an intravenous dose reaches the
urine.7-9 The high proportion of hepatic elimination
with torsemide predicts that there should be a decrease
in total body clearance of torsemide in patients with
hepatic disease or other disorders impairing hepatic
metabolism. Thus, in patients with cirrhosis, this decreased clearance may allow more drug to be available
to reach the kidney to exert the desired diuretic effect,
as has been observed with xipamide.10 The increased
delivery of torsemide to its urinary site of action could
offset the diminished pharmacodynamics of response.
One goal of this study was to test this hypothesis.
CLINICAL PHARMACOLOGY & THERAPEUTICS
VOLUME 54, NUMBER 1
Schwartz et al.
91
Table I. Demographic data
Patient
No.
1
2
3
4
5
6
7
8
9
10
11
12
Sex
Male
Male
Male
Male
Male
Male
Male
Male
Male
Male
Male
Female
Age Weight
(yr) (kg)
63
41
53
41
41
60
44
53
66
45
59
38
88
90
88
74
88
76
99
62
69
65
85
120
Creatinine
clearance
(ml/min)
63
79
106
105
123
76
99
111
100
127
65
124
METHODS
Study design. This study was a two-center, randomized, open-label, two-treatment, two-sequence
crossover design in a group of patients with ascites
caused by hepatic cirrhosis. We examined the bioavailability, pharmacokinetics, and pharmacodynamics of torsemide. The parameters obtained in the subjects with cirrhosis were compared with data obtained
from normal volunteers in an earlier study that used a
similar protocol.11
Patients. Thirteen patients (12 men and one
woman) with ascites caused by cirrhosis were enrolled, and 12 patients completed the study. Patient
characteristics are shown in Table I. All patients had
confirmed or suspected diagnoses of chronic, stable
cirrhosis based on histories, physical examinations,
and laboratory tests (liver biopsy was not a prerequisite). All patients had either ascites or a history of ascites. Subjects were excluded if they had acute hepatic
encephalopathy, gastrointestinal hemorrhage within 3
months of study entry, or other serious abnormalities
other than those related to cirrhosis. Women of childbearing potential were also excluded if they were not
postmenopausal or surgically sterile. Before participation, each patient gave informed consent in accordance with the standards of the Indiana UniversityPurdue University at Indianapolis Institutional Review
Board or the Institutional Review and Research Committee of Meridia Huron Hospital, Cleveland, Ohio.
Experimental protocol. Patients were screened,
gave written informed consent, and were admitted to
the clinical research center. All subjects received a
metabolic diet containing 80 mEq sodium and 60 to
80 mEq potassium per day. Diuretics, excluding spironolactone, which had been administered at a con-
Fig. 1. Plasma concentrations (top panel) and urinary excretion rates (bottom panel) versus time after intravenous
and oral administration of 10 mg torsemide to patients with
cirrhosis.
stant dose rate for at least 7 days, were discontinued.
The low sodium intake was intended to prevent any
loss of blood pressure control or fluid accumulation
while thiazide or loop diuretics were discontinued. Patients equilibrated on the diet for 5 days, during which
daily 24-hour urine and blood specimens were collected for electrolytes (sodium, chloride, and potassium) and creatinine. After establishment of sodium
balance, patients were randomized on day 6 to receive
either one 10 mg tablet by mouth or 10 mg torsemide
by intravenous infusion over 30 minutes.
After an overnight fast (minimum of 10 hours),
those patients randomized to receive the oral
torsemide tablet were given the study drug with 240
CLINICAL PHARMACOLOGY & THERAPEUTICS
JULY 1993
92 Schwartz et al.
Table II. Mean ± SD pharmacokinetic parameters of
torsemide after intravenous and oral administration of
10 mg to patients with cirrhosis
Parameter
Intravenous
Cmax (g/ml)
tmax (hr)
AUC (hr •g/ml)
t1/2 (hr)
CL (ml/min)
CLR (ml/min)
CLNR (ml/min)
Varea (L)
Ae (%dose)
ERmax (g/min)
Oral
1.7 ± 0.4
0.52 ± 0.07
4.81 ± 1.56
8.07 ± 3.38
38.3 ± 13.1
9.1 ± 3.4
29.2 ± 13.2
24.0 ± 6.2
26.6 ± 10.7
18.1 ± 6.1
1.45 ± 0.4
0.71 ± 0.18
4.49 ± 1.45
7.49 ± 2.6
—
10.8 ± 4.3
—
—
26.9 ± 11.6
14.7 ± 6.1
p Value
0.012
0.008
0.576
0.664
—
0.583
—
—
0.919
0.133
Cmax, Maximum plasma concentration; t max, time to reach Cmax; AUC,
area under the plasma concentration-time curve; t1/2, half-life; CL, total
clearance; CLR, renal clearance; CLNR, nonrenal clearance; Varea, volume of
distribution; Ae, amount of torsemide excreted into the urine; ER max, maximal urinary torsemide excretion rate.
Table IV. Comparison of torsemide pharmacokinetic
parameters after intravenous and oral administration of
10 mg to patients with cirrhosis and to healthy
subjects
Parameter
Parameter
Intravenous
Oral
Na+ (mEq)
CF- (mEq)
K+ (mEq)
Urine (ml)
233 ± 83 261 206 ± 76
± 53 78 ± 36 264 ± 95 73
3846 ± 1392
± 37 3958 ±
1329
p Value
0.412
0.876
0.225
0.748
Na+, Sodium; Cl- , chloride; K+, potassium.
ml water. Patients remained fasting for 4 hours after
drug administration. Standard meals were provided at
noon and 5 PM. Those patients randomized to receive
the intravenous injection received torsemide over 30
minutes in 50 ml of 5% dextrose in water by way of
peripheral vein with an infusion pump. In both study
phases, plasma samples were obtained for measurement of electrolytes, creatinine, and torsemide before
and 15, 30, 45, 60, 75, and 90 minutes and 2, 3, 4, 6,
8, 12, 16, 24, 30, and 36 hours after dosing. Urine
samples for similar measurements were obtained by
spontaneous voiding at 1/2, 1, 11/2, 2, 3, 4, 5, 6, 8,
12, 24, and 36 hours after administration of the drug.
To prevent volume depletion and to maintain diuretic responsiveness during the study periods, patients had urinary output replaced volume per volume
plus 1 ml/min intravenously with one-half normal saline solution from the start of drug administration
through 8 hours after dosing. On the day after drug
administration (days 7 and 1 2), patients were assessed
for net sodium balance bv oomparison of 24-hour uri-
Healthy
subjects
Oral
Cmax (g/ml)
t max (hr)
AUC (hr • g/ml)
t1/2 (hr)
CLR (ml/min)
Ae (%dose)*
1.45 ± 0.4
0.71 ± 0.18
4.5 ± 1.4
7.5 ± 2.6
10.8 ± 4.3
26.9 ±11. 6
1.27 ± 0.13
0.86 ± 0.18
3.7 ± 1.7
3.5 ± 1.2
7.2 ± 3.2
14.9 ± 6.5
4.8 ± 1.6
8.1 ± 3.4
38.3 ± 13.1
9.1 ± 3.4
29.2 ± 13.2
24.0 ± 6.2
26.6 ± 10.7
18.1 ± 6.1
4.5 ± 2.2
3.6 ± 1.9
43.0 ± 13.8
6.4 ± 2.1
36.6 ± 12.7
11.7 ± 3.5
15.7 ± 4.9
11.5 ± 5.2
Intravenous
AUC (hr • g/ml)
t1/2 (hr)
CL (ml/min)
CLR (ml/min)
CLNR (ml/min)
Varea (L)
Table III. Mean ± SD cumulative 24-hour excretion
of electrolytes and urine after intravenous and oral
administration of 10 mg torsemide to patients with
cirrhosis
Patients with
cirrhosis
Ae (%dose)
ERmax (g/min)
nary excretion of sodium to total sodium intake, and
any net loss was replaced with intravenous normal saline solution. Patients then reattained sodium equilibrium on the metabolic diet for 3 days. On day 11, subjects underwent the second single-dose study phase
identical to that described above. After a physical examination was conducted and laboratory tests were
obtained, subjects were discharged from the clinical
research center on the morning of the thirteenth day.
Laboratory determinations. Plasma and urine concentrations of torsemide and metabolites were determined by an HPLC assay. 12 Sodium and potassium
concentrations were determined by flame photometry,
and chloride concentrations were determined by chlorimetry. Creatinine concentrations were measured
with use of the Jaffe reaction.
Data analysis. Pharmacokinetic parameters for
torsemide were determined with standard methods. 13
The maximum plasma concentration (C max) and time to
reach Cmax (tmax) were determined by direct observation of
the data. The terminal elimination rate constant (k e )
was calculated from the negative of the slope of the
terminal
log-linear
portion
of
the
plasma
concentration-time curve by use of linear regression
of the natural logarithm of plasma concentration
against time. Half-life (t1/2) was calculated as 0.693 divided by the terminal elimination rate constant. The
areas under the plasma concentration-time curve
(AUC) and the first moment curve (AUMC) to the final measurable sample were calculated by use of the
CLINICAL PHARMACOLOGY & THERAPEUTICS
VOLUME 54, NUMBER 1
Schwartz et al.
93
Fig. 2. Urinary excretion rates of electrolytes and urine after intravenous and oral administration
of 10 mg torsemide to patients with cirrhosis.
trapezoidal method and extrapolated to infinity with
the final observed plasma concentration and ke. Renal
clearance (CLR) was calculated as the total amount of
unchanged drug excreted into the urine divided by the
AUC extrapolated to infinity. The urinary excretion
rate of torsemide over each collection interval was calculated by dividing the amount of torsemide excreted
during the interval by the duration of the interval. The
maximum excretion rate (ERmax) was determined by
observation. For the intravenous treatment, total clearance (CL) was calculated by dividing the dose by
AUC, and volume of distribution (V area was calculated by dividing the dose by the product of AUC and
ke. Nonrenal clearance (CLNR) was the difference between CL and CLR. Bioavailability was estimated as
the ratio of the AUC after oral administration to that
after intravenous administration. Mean residence time
(MRT) was calculated by dividing the AUMC by
AUC, and mean absorption time (MAT) was the difference between MRToral, and MRTiv.
Fig. 3. Relationship between urinary sodium excretion and
torsemide excretion rates after intravenous and oral administration of 10 mg to patients with cirrhosis. Each point represents the mean value in a collection interval, and the curve
represent, the line of best fit.
94 Schwartz et al.
CLINICAL PHARMACOLOGY & THERAPEUTICS
JULY 1993
Fig. 4. Comparison of plasma concentrations and urinary excretion rates versus time after intravenous and oral administration of 10 mg torsemide to patients with cirrhosis and to healthy subjects.
Drug effect was assessed as net cumulative electrolyte excretion and by the relation between the urinary
diuretic excretion rate and the natriuretic response.
The latter method was used because the site of action
of torsemide is the luminal (urinary) side of the
nephron,5 and previous studies have shown that this
relationship accurately quantifies the pharmacodynamics of response to loop diuretics.1,5,14
For some treatments in some patients, the terminal
torsemide concentration-time data did not allow estimation of ke, and thus AUC could not be calculated;
all other data were used in the analysis. Comparisons
between oral and intravenous studies were by
ANOVA. No center effect was discerned. Approximate 90% confidence intervals on absolute bioavailability for the treatment mean ratio of oral AUC to intravenous AUC for torsemide were constructed with
the two one-sided tests procedure.
RESULTS
Patients with cirrhosis. Overall, the drug was well
tolerated, with 12 of the 13 patients completing the
study. One patient was withdrawn from the study before the second single-dose study day because of fluid
and electrolyte abnormalities attributed to the low sodium diet and continued spironolactone administration. Data from this patient were not included in the
calculations.
Plasma concentration-time and urinary excretion
rate- time curves for the two routes of administration
were essentially the same (Fig. 1). There were no significant differences among those parameters common
to both routes of administration (AUC, t 1/2, CLR,
amount excreted, and ERmax; Table II).
Torsemide was rapidly absorbed after oral administration, reaching peak concentrations in less than an,
hour. The absolute bioavailability was 96%, with con-
CLINICAL PHARMACOLOGY & THERAPEUTICS
VOLUME 54, NUMBER 1
Schwartz et al. 95
Fig. 5. Comparison of the relationship between urinary sodium excretion and torsemide excretion rates after intravenous and oral administration of 10 mg torsemide to patients
with cirrhosis and to healthy subjects. Each point represents
the mean value in a collection interval, and the curve represents the line of best fit.
fidence limits (two one-sided tests at = 0.05) of
84% to 109%. Comparison of MRT values between
oral and intravenous administration (6.97 ± 2.69
hours and 6.16 ± 2.97 hours, respectively) showed a
very short MAT (0.79 ± 1.63 hour). Comparison of
this value to MRTiv indicates that torsemide does not
follow absorption-limited kinetics in patients with cirrhosis. This finding is in contrast to furosemide,
which obeys absorption-limited kinetics in both
healthy subjects15 and in patients with cirrhosis.16
Due to the higher maximum urinary excretion rate
of torsemide after intravenous administration, the
maximum electrolyte excretion and urinary output
rates were higher, but the time course and the cumulative 24-hour excretions of electrolytes and urine were
similar for the two routes of administration (Table III;
Fig. 2). The relationship between urinary torsemide
excretion rate and urinary sodium excretion rate is depicted in Fig. 3. The 10 mg dose of torsemide was not
sufficient to reach the upper plateau of the pharmacodynamic curve. However, because a vigorous diuresis
should be avoided in patients with cirrhosis, we did
not consider administering larger doses.
DISCUSSION
With the expected exception of peak concentration
and time to peak concentration, the pharmacokinetics
for intravenous and oral administration of torsemide in
Fig. 6. Comparison of urinary sodium excretion rates versus
time after intravenous (top panel) and oral (bottom panel)
administration of 10 mg torsemide to patients with cirrhosis
and to healthy subjects.
patients with cirrhosis were similar (Fig. 1; Table II).
The high bioavailability observed in patients with cirrhosis was greater than the approximate 80% observed
in healthy subjects,12 possibly because of decreased
first-pass elimination through the diseased liver.
Except for a higher peak concentration after intravenous administration in healthy subjects (attributable to
a 3-minute rather than 30-minute infusion time), mean
intravenous and oral plasma concentration-time
curves were similar (Fig. 4, upper panels). Table IV
lists selected pharmacokinetic parameters from this
study and comparable values in healthy subjects.12
Cirrhosis did not appear to affect the rate of absorption of torsemide after oral administration because
CLINICAL PHARMACOLOGY & THERAPEUTICS
96
Schwartz et al.
both Cmax and tmax values were comparable to those in
normal subjects.12 This is somewhat surprising because delayed absorption of furosemide has been observed in other edematous disorders, including cirrhosis.16 There is a greater excretion rate of torsemide
into the urine of patients with cirrhosis (Fig. 4, lower
panel) corresponding to an increased renal clearance
in these patients (Table IV). As such, the fraction of
dose reaching the urinary site of action is about 70%
higher than in healthy subjects (Table IV).
The Varea in the patients with cirrhosis averaged approximately twofold greater than that in normal subjects (Table IV). This is most likely because of diminished protein binding of torsemide in liver disease.
Because CL was little changed, the greater V area in
patients with cirrhosis resulted in a doubling of elimination t1/2.
A decrease in CLNR was expected in a patient population with impaired liver function. Even though
CLNR appears to be somewhat less in patients with
cirrhosis compared with healthy subjects, this value
may underestimate the degree of diminished hepatic
metabolism of torsemide. The diminished protein
binding of torsemide would ordinarily be expected to
increase CLNR and CL. Thus the decrease in CLNR of
total torsemide likely represents a substantial reduction in elimination of unbound torsemide.
Even though renal function in our patients was
comparable to if not less than that of healthy subjects,
CLR was-greater in patients with cirrhosis. Theoretically, this increase in renal clearance could be explained by diminished plasma protein binding of
torsemide, allowing more drug to be filtered at the
glomerulus. Quantitatively, however, this effect is
probably small because the majority of the renal elimination of torsemide occurs by way of tubular secretion
at the organic acid secretory pathway of the proximal
tubule. 5 The related sulfonyl diuretic furosemide has
been shown to exhibit low extraction or diffusionlimited kinetics with respect to tubular secretion. 17
One would expect that a decrease in protein binding of
a drug that had this property would increase tubular
secretion and hence CLR. The same may also apply to
torsemide. The overall result of the pharmacokinetic
alterations of torsemide in patients with cirrhosis compared with normal subjects is that a greater percentage
of the dose is delivered to the renal site of action over
a more prolonged period (Table IV; Fig. 4).
Diuretic resistance, consistent with hyperaldosteronism,18 is shown by a shift to the right of the pharmacodynamic curve in patients with cirrhosis (Fig. 5).
It is interesting that this feature occurred in our study
JULY 1993
in spite of ongoing spironolactone administration. In
spite of this pharmacodynamic difference, the natriuretic response in patients with cirrhosis was essentially the same as that in healthy subjects (Fig. 6). In
turn, overall natriuresis after a 10 mg intravenous dose
was similar (233 versus 262 mEq sodium/24 hours,
respectively).
Thus the pharmacokinetic alterations in cirrhosis
overcame to a considerable degree the pharmacodynamic alterations, resulting in a similar net natriuresis.
The approximate twofold increase in the CL R of
torsemide in patients with cirrhosis resulted in greater
amounts of urinary torsemide. Consequently, even
though the amount of sodium excreted per unit of
torsemide is less in patients with cirrhosis, the overall
natriuretic effect is very similar to that which occurs
in healthy subjects.
References
1. Brater DC. Resistance to loop diuretics, why it happens
and what to do about it. Drugs 1985;30:427-43.
2. Neugebauer G, Besenfelder E, and von Mollendorff E.
Pharmacokinetics and metabolism of torasemide in
man. Arzneimittelforschung 1988;38:164-6.
3. Lesne M, Clerckx-Braun F, Duhoux P, van Ypersele de
Strihou CH. Pharmacokinetics of a new diuretic, torasemide, in man. Arch Int Pharmacodyn 1981;249:
322-5.
4. Lesne M, Clerckx-Braun, Duhoux P, van Ypersele de
Strihou CH. Pharmacokinetic study of torsemide in humans: an overview of its diuretic effect. Int J Clin Pharmacol Ther Toxicol 1982;20:382-7.
5. Brater DC, Leinfelder J, Anderson SA. Clinical pharmacology of torasemide, a new loop diuretic. CLIN
PHARMACOL THER 1987;42:187-92.
6. Lesne M. Comparison of the pharmacokinetics and
pharmacodynamics of torsemide and furosemide in
healthy volunteers. Drug Res 1988;38:160-3.
7. Pentikainen PJ, Neuvonen PJ, Kekkim, Penttila A.
Pharmacokinetics of intravenously administered bumetanide in man. J Pharmacokinet Biopharm 1980;8:21928.
8. Fuller R, Hoppel C, Ingalls ST. Furosemide kinetics in
patients with hepatic cirrhosis with ascites. CLIN PHAR
MACOL THER 1981;30:461-7.
9. Keller E, Hoppe-Seyler G, Mumm R, Schollmeyer P.
Influence of hepatic cirrhosis and end-stage renal dis
ease on pharmacokinetics and pharmacodynamics of fu
rosemide. Eur J Clin Pharmacol 1981;20:27-33.
10. Hammerlund MM, Paazlow LK. Odlind B. Pharmacokinetics of furosemide in man after intravenous and oral
administration. Application of moment analysis. Eur J
Clin Pharmacol 1984;26:197-207.
11. Barr WH, Smith HL, Karnes HT, et al.Torasemide
dose-proportionality of pharmacokinetics and pharma-
CLINICAL PHARMACOLOGY & THERAPEUTICS
VOLUME 54, NUMBER 1
codynamics. In: Kriick F, Mutschler E, Knauf H, eds.
Torasemide—clinical pharmacology and therapeutic
applications. Proceedings of the International Symposium on Torasemide, Munich, October 21-23, 1988.
Progress in pharmacology and clinical pharmacology
series, vol 8/1. New York:Gustav Fischer Verlag,
1991:29-37.
12. March C, Farthing D, Wells B, Besenfelder E, Karnes
HT. Solid-phase extraction and liquid chromatography
of torsemide and metabolites from plasma and urine. J
Pharm Sci 1990;79:453-7.
13. Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New
York:Marcel Dekker, 1982:45-111.
14. Smith D. Loop diuretics: effects in an eliminating or
gan. In: Smith RB, Kroboth PD, Juhl RP, eds., Phar
macokinetics and pharmacodynamics, vol 2. Current
problems, potential solutions. Cincinnati: H. Whitney
Books, 1988:107-38.
Schwartz et al.
97
15. Knauf H, Gerok W, Mutschler E, Scholmericb J,
Spahn H, Wietholtz H. Xipamide disposition in liver
cirrhosis. CLIN PKARMACOL THER 1990;48:628-32.
16. Fredrick MJ, Pound DC, Hal] SD, Brater DC. Fu rosemide absorption in patients with cirrhosis. CLIN
PHARMACOL THER 1991;49:241-7.
17. Hall SD, Rowland M. Influence of fraction unbound
upon the renal clearance of furosemide in the isolated
perfused rat kidney. J Pharmacol Exp Ther
1985;232:263-8.
18. Perez-Ayuso RM, Arroyo V, Planas R, et al. Random
ized comparative study of efficacy of furosemide versus
spironolactone in nonazotemic cirrhosis with ascites.
Relationship between the diuretic response and the ac
tivity of the renin-aldosterone system. Gastroenterology
1983;84:961-8.