Preparation of bimetallic catalysts using continuous processing
advertisement

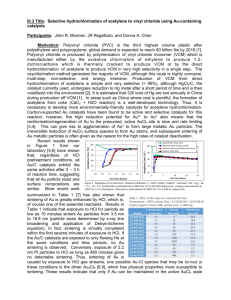
Title: Preparation of bimetallic catalysts using continuous processing methods Participants: John R. Monnier, Akkarat Wongkaew, and D.A. Blom Motivation: Electroless deposition is a method of preparing bimetallic catalysts in which only bimetallic particles of known composition are produced [1-4]. Batch methodology has been primarily used thus far for preparing such catalysts. This method has several drawbacks: (1), only relatively small amounts of catalyst can be prepared at one time and/or (2), only relatively low levels of the second metal (M) can be deposited on the primary metal (A). This is because all of the reducing agent (RA) and reducible metal salt must be added into the vessel before ED begins. ED is governed by a series of kinetic reactions: (1), R thermal rxn = ko exp(-Ea/RT) [CRA]x [CM salt]y. (2), R cat dep = ko’ exp(-Ea’/RT) [CRA]a [CM salt]b [CA sites]c. (3), R autocat dep = ko” exp(-Ea”/RT) [CRA]d [CM salt]e [CM sites]f. The thermal reaction (1), or the non-catalytic reduction of the reducible metal salt, is thus a function of the concentrations of reducing agent and reducing metal salt. In the batch method, both of these components must be front-loaded and thermal stability in the high initial concentrations of RA and reducible metal salt often becomes the critical step in the ED process. Further, the rates of catalytic (2) and autocatalytic (3) deposition are strong functions of the concentrations of reducing agent and reducible salt concentrations, meaning the rates are much higher at the beginning of the deposition process than at the end. This can result in heterogeneities in the position of the deposited metal. Finally, whether or not catalytic deposition (on A sites) or autocatalytic deposition (on just-deposited M sites) occurs can be a function of the concentration of reducible metal salt M in solution [5-7]. Continuous addition of the reducing agent and/or reducible metal salt minimizes the thermal stability issue and also provides a method for commercial, larger-scale production of bimetallic catalysts. Hypothesis: Continuous ED methods can be used to synthesize high loadings of ED metals and large quantities of bimetallic catalysts. Micro-processor syringe pumps will be used to add controlled amounts of reducing agents and/or reducible metal salts to a vessel containing an aqueous mixture of a supported, monometallic catalyst to prepare bimetallic catalysts containing high amounts of the second metal. Continuous methods are also more amenable to on-line monitoring of the deposition process, an important factor in largescale production. Preliminary work by co-PI Wongkaew for the preparation of high weight loadings (5 – 25 wt%) of Pt on 30 wt% Pd/C have indicated the syringe pump apparatus in Figure 1 works well for deposition of high weight loadings of a second metal. The UV-visible spectra for the PtCl62- complex (at 295 nm) [8] shown in Figure 2 shows that the addition of ethylene diamine (EN) as a complexing agent for PtCl62is required for thermal stability in the presence of hydrazine (N2H4) as a reducing agent. At a mole ratio of [N 2H4]/[PtCl62-] = 5:1, bath lifetime is increased from 12 to 26 min when a 2 : 1 molar excess of [EN]/[PtCl62-] is added to the bath. It also underscores the need to continuously and slowly add N2H4 to the bath so that unwanted thermal reduction of PtCl62- does not occur. Finally, it indicates that UV-visible spectroscopy can be used as a real-time probe to monitor the rate of continuous, electroless deposition, an important feature for large-volume catalyst production. Up to this point, samples have periodically been taken from the ED bath, filtered, and then analyzed by atomic absorption spectroscopy. We have recently designed a continuous-flow UV cell that employs a pump and fritted filter (to keep the particulate catalyst in the ED bath) to successfully circulate the solution between the UV cell and the ED bath. The results in Figure 3 show kinetic curves for deposition of high weight loadings of Pt on 30 wt% Pd/C (Dispersion of Pd = 25%; dPd = 4.5 nm). In this series of experiments, the N2H4 solution was pumped at 1.67 ml/min into a bath containing all other components. Since bath stability is typically controlled by the concentration of reducing agent, particularly for active reducing agents, it was sufficient to pump only N2H4 to a bath already containing the PtCl62-. The curve for the Pd-free (blank) sample indicates that thermal stability was only ~20 minutes before thermal reduction of PtCl62 Pdo occurred. Regardless, deposition of 11.7%, 17.2%, and 22.7 wt% Pt readily occurred within this time frame. Deposition of these amounts of Pt would not have been possible using batch methods, where all N2H4 would have been added at the beginning of the deposition experiment. In order to determine the morphology of Pt deposition, STEM analysis (by co-PI Blom) was conducted using an aberration-corrected JEOL 2100F scanning transmission electron microscopy (STEM) and Z-contrast imaging. The images for 17.2 wt% Pt deposited on 30 wt% Pd/C in Figure 4 show clearly that a shell of Pt as a (111) surface layer has been uniformly-deposited on the Pd core. Because of the relatively shallow depth of field at this high magnification, only one of the core-shell particles is well focused. The well-ordered Pt shell structures and the fact that all particles exist as Pd core-Pt shell structures (many other areas of the surface were also imaged) show the selectivity of this ED process. Energy dispersive x-ray spectroscopy (EDS) analysis was conducted on the particle shown in Figure 5 and the atomic Pt and Pd positions are presented in Figure 6. The Pt and Pd x-ray intensities show conclusively that bimetallic particles with Pt shell thickness of approximately 1.5 nm on a Pd core of about 4 – 6 nm diameter have been formed, which is in good agreement with 2.7 ML thickness of Pt on 4.5 nm Pd particle diameters. This control of bimetallic structure at high loadings of deposited metal also validates this Figure 5. EDS image area for particle of 17.2% Pt-30 type of continuous wt% Pd/C sample. particle synthesis. Objective: Evaluate continuous ED protocols for different combinations of reducing agents and reducible metal salts to optimize continuous ED methods for high deposition levels and large quantities of bimetallic catalysts. Develop a facile method for continuous monitoring of the deposition process using UVvisible spectroscopy, rather than analysis by atomic absorption of aliquots of the ED bath at discrete time intervals. The ultimate goal is to develop a continuous methodology that can be used commercially for production of bimetallic catalysts. Research Plan: We will use the continuous ED set-up shown in Figure 1. If it is desirable to examine simultaneous deposition of two metals to the base monometallic catalyst, a third syringe pump will be installed. Parameters to be examined will include pump rates, deposition temperature, pH values, and addition of organic stabilizers. Before deposition experiments are conducted, it will be necessary to determine thermal stabilities of the bath as a function of pumping rates of reducing agent and/or reducible metal salt. For reducible metal salts with marginal thermal stability, stabilizing agents such as alkylamines or organic acid salts will be used; the stabilizers function primarily as ligands to lower reduction rates of the coordinated metal ion. The kinetics in Figure 2 indicate that ethylenediamine (EN) was required for stability, even when the very active N2H4 was being slowly pumped into the ED vessel over a 30 min interval. Bimetallic catalysts will not be limited to core-shell structures, but will include controlled compositions with both metals at the surface. First year milestone: Several protocols for addition of high loadings of reducible metals such as Pt, Ru, and Co to supported monometallic catalysts will be developed. Deposition will be extended to extruded supports. Multiple gram amounts will be prepared. Cost: $70,000 for year one and successive years at $60,000/yr. First year includes $10,000 for chemicals, pumps, and computers. References 1. K.D. Beard, D. Borelli, A.M. Cramer, D. Blom, J.W. Van Zee, and J.R. Monnier, “Preparation and structural analysis of carbon-supported Co core/Pt shell electrocatalysts using electroless deposition methods,” ACS Nano, 3, 2841 (2009). 2. Rebelli,, A.A. Rodriguez, S. Ma, C.T. Williams, J.R. Monnier, “Preparation and characterization of silica-supported,Group IB-Pd bimetallic Catalysts prepared by electroless deposition methods,” Catal. Today, 160 (2011) 170. 3. A.A. Rodriguez, C.T. Williams, and J.R. Monnier, “Selective liquid-phase oxidation of glycerol over Au-Pd/C bimetallic catalysts prepared by electroless deposition,” Appl. Cat. A: General, 475 (2014) 161. 4. Y. Zhang, W. Diao, C.T. Williams, and J.R. Monnier, “Selective hydrogenation of acetylene in excess ethylene using Ag- and Au-Pd/SiO2 bimetallic catalysts prepared by electroless deposition,” Appl. Cat. A: General, 469 (2014) 419. 5. I. Ohno, “Electrochemistry of electroless plating,” Mat. Sci. Engr., A146 (1991) 33. 6. S.S. Djokić, “electroless deposition of metals and alloys,” Mod. Aspects Electrochem, 35, (2002) 51. 7. M.T. Schaal, A.C. Pickerell, C.T. Williams, and J.R. Monnier, “Characterization and evaluation of Ag-Pt/SiO2 catalysts prepared by electroless deposition,” J. Catal., 254 (2008) 131. 8. L. Obreja, D. Pricop, N. Foca, and V. Melnig, “Platinum nanoparticles synthesis by sonochemical methods,” Materiale Plastice, 47 (2010) 42.