THROMBOTIC DISORDERS: Basic mechanisms, classification and
advertisement

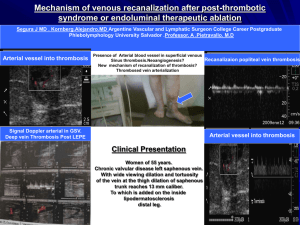
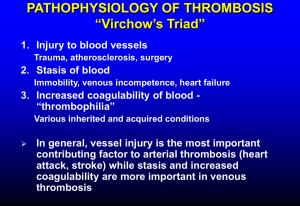
Thrombotic Disorders 1 THROMBOTIC DISORDERS A thrombotic disorder is an acquired or hereditary disorder predisposing to the unnecessary formation of a thrombus (clot), which is the end result of activation of the coagulation cascade. So in essence, a thrombotic disorder results either from the undue activation or lack of inhibition of the coagulation cascade. REGULATION OF THROMBOSIS: Since thrombus formation starts from the formation of a platelet plug at the site of vascular injury followed by deposition of fibrin as a result of activation of the coagulation cascade, inhibitory factors exist at both levels that limit the formation of a thrombus beyond what is physiologically required by the body. INHIBITION OF PLATELETS: As soon as an adequate platelet plug is formed, APD and thromboxane A2 are dissipated in the circulation limiting the size of the thrombus physiologically required. In addition, direct inhibition of the platelets by ADPase, prostacyclin, and nitric oxide is achieved. INHIBITION OF COAGULATION CASCADE: The control of the coagulation cascade occurs at multiple levels: 1. Inhibition of thrombin by a protein called anti-thrombin III (AT) 2. Inactivation of activated factor V (FVa) and VIII (FVIIIa) by activated protein C (APC) and protein S inhibition pathway 3. Tissue factor pathway inhibition (TFPI) 4. Fibrinolysis The contents & pictures in this handout are derived from various sources including books, journal articles and patient material for teaching purposes only. No commercial incentives are sought or intended. Thrombotic Disorders 2 ANTITHROMBIN: Antithrombin III (AT-III) is a plasma proteinase inhibitor synthesized in the liver that inactivates thrombin and other enzymes responsible for the generation of thrombin. The name antithrombin is somewhat misleading as it implies that this protein inhibits only thrombin when in fact it inhibits many other activated coagulation proteins including factors Xa, IXa, XIa, XIIa, and pre-kallikrein-HMWK in the intrinsic pathway and VIIaTF in the extrinsic pathway. This combination of being able to block thrombin-mediated fibrin formation and inhibit enzymes in the pathways responsible for prothrombin activation makes AT-III a powerful and important endogenous anticoagulant molecule and explain why patients with even modest AT-III deficiencies may experience clinical thrombosis. [This picture is adapted from Hemostasis & Thrombosis by Colman et al. 4th edition. Lippincott-Williams & Wilkins] Antithrombin III inhibits its target protein substrates by direct binding. This inhibition is very efficient in the presence of cofactors such as heparin cofactor II (HcII) in blood. Endothelial cell surface and subendothelial matrix contain heparan sulfate proteoglycans (HSPGs) that bind to antithrombin III and form a complex on their surface. When after vascular injury thrombin is formed AT-III in complex with HSPGs binds to thrombin and other coagulation factors leading to their clearance. Circulating AT-III in complex with HcII does the same job at the site of clot formation. Antithrombin III in complex with thrombin and other activated coagulation factors is cleared from circulation by liver. TISSUE FACTOR PATHWAY INHIBITOR (TFPI): Tissue factor pathway inhibitor (TFPI) is a plasma proteinase inhibitor that regulates tissue factor-mediated coagulation by producing factor Xa-dependent feedback inhibition of factor VIIa-Tissue factor (VIIIa-TF) complex. The inhibition occurs in 2 steps: first, TFPI binds to Xa and inactivates it then in the second step TFPI-Xa complex binds to VIIa-TF complex and causes its inhibition resulting in the lack of activation of the coagulation cascade by the extrinsic pathway. The contents & pictures in this handout are derived from various sources including books, journal articles and patient material for teaching purposes only. No commercial incentives are sought or intended. Thrombotic Disorders 3 ACTIVATED PROTEIN-C (APC) AND PROTEIN-S PATHWAY: Two proteins called protein C and protein S, both synthesized in liver and vitamin Kdependent, are an important mechanism in the control of thrombus formation. Protein C, a serum protease, binds to another protein called thrombomodulin (TM) in complex with thrombin (T) and also binds to its receptor on the endothelial cell surface (EPCR) and becomes activated. The activated protein C (APC) cleaves activated coagulation factors V (FVa) and VIII (FVIIIa) with the help of its non-enzymatic cofactor, protein S. In fact, protein S can independently cleave Va, VIIIa and also Xa. About 60% of the protein S is inert and bound to C4BP, a component of the complement system and the remaining 40% is free and is the active form. The cleavage of activated factor V and VIII to their inactivated forms (Vi & VIIIi) takes place on the lipid bi-layer surface of platelets. [This picture is adapted from BLOOD: Principles & Practice of Hematology by Handin et al. Lippincott Press] The contents & pictures in this handout are derived from various sources including books, journal articles and patient material for teaching purposes only. No commercial incentives are sought or intended. Thrombotic Disorders 4 CLASSIFICATION OF THROMBOTIC DISORDERS: A hypercoagulable state is suspected in a patient with a documented thrombotic disorder with the following presentations: Age below 40 Venous thrombosis in unusual sites such as (a) Cerebral veins and sinuses (b) Retinal vein thrombosis (c) Renal vein thrombosis (d) Venous thrombosis in portal venous system including mesenteric veins, splenic veins, portal vein, hepatic vein Recurrent thrombosis Family history of thrombosis Repeated thrombosis despite anticoagulation therapy Recurrent fetal loss; HELLP syndrome Apparently unprovoked thrombosis Hypercoagulable states can be divided into two general categories: 1. Inherited disoders (i) Antithrombin III deficiency or dysfunction (ii) Protein C deficiency (iii) Protein S deficiency (iv) Resistance to activated protein C by factor V (factor V Leiden) (v) Prothrombin polymorphism (G20210A) (vi) Heparin cofactor II deficiency (vii) Dysfibrinogenemia –such as fibrinogen Marburg, fibrinogen Paris V (viii) Hyperhomocysteinemia – associated with arterial thrombosis 2. Acquired disorders and hypercoagulable states (i) Antiphospholipid syndromes – discussed later (ii) Paroxysmal Nocturnal Hemoglobinuria (PNH) – cerebral vein thrombosis is a feature (iii) Myeloproliferative disorders – increased risk for portal venous system thrombosis (iv) Acute promyelocytic leukemia – cells release procoagulant proteins (v) Mucin-secreting adenocarcinomas – (vi) Nephrotic syndrome – increased renal loss of anticoagulant proteins (vii) Hyperviscosity syndromes – Waldenstrom’s macroglobulinemia Hyperlipidemia – in particular with type II hyperbetalipoproteinemia (viii) Pregnancy & high estrogen states – complex mechanisms (ix) Immobilization – venous stasis predisposing to formation of thrombus (x) Heparin induced thrombocytopenia syndrome – discussed elsewhere (xi) Thrombotic thrombocytopenic purpura – discussed elsewhere (xii) Disseminated intravascular coagulation (DIC) – discussed elsewhere (xiii) Intravascular devices (xiv) A variety of other conditions (Behcet’s syndrome, ulcerative colitis) The contents & pictures in this handout are derived from various sources including books, journal articles and patient material for teaching purposes only. No commercial incentives are sought or intended. Thrombotic Disorders 5 INHERITED AND ACQUIRED THROMBOTIC DISORDERS Acquired disorders are more commonly associated with venous thromboembolism (VTE). Nevertheless, hereditary predisposition is increasingly being identified in, at least, some of the patients thought to have VTE as a result of an acquired disorder. Also, spontaneous VTE in the absence of an identifiable acquired disorder is an area of intense research because in a large proportion of individuals with an arterial or venous thrombosis no known genetic predisposition to VTE could be demonstrated. HEREDITARY RISK FACTORS OF THROMBOPHILIA: ACTIVATED PROTEIN C (APC) RESISTENCE / FACTOR VLEIDEN MUTATION: This is the most common of all known risk factors for hereditary venous thrombophilia. As you learned previously that activated protein C (APC) is required for inactivation of activated coagulation factors V (FVa) and factor VIII (FVIIIa) such that resistance of factor V to cleavage by protein C results in an undue prolonged activation of factor V favoring a thrombophilic state. This resistance is most commonly caused by a single base substitution at position 1691 (G to A) which results in the formation of a mutated factor V called factor VLeiden (First described in Leiden, Netherland) in which the normal Arginine amino acid at position 506 is replaced by Glutamine (Arg506 to Gln). This mutated factor VLeiden is resistant to cleavage by APC. Resistance of factor V to APC has also been seen with other mutations in factor V (Factor VCambridge). The normal activation and inactivation of factor V is shown below: Fig. The top part shows a factor V molecule that is cleaved at three different sites (709, 1018 & 1545) by thrombin or activated factor X (Xa) for full cofactor activity (Va). After cleavage heavy and light chains are held together by a metal ions (Me). After serving as an activated cofactor, the activated factor V (Va) is physiologically shut down by cleavage by protein C at site 306 & 506 releasing inactivated factor V (iVa). [This picture is adapted from Williams Hematology by Beutler et al. 6th edition. McGraw Hill Press] The contents & pictures in this handout are derived from various sources including books, journal articles and patient material for teaching purposes only. No commercial incentives are sought or intended. Thrombotic Disorders 6 ATTRIBUTES OF FACTOR VLEIDEN MUTATION: Occurrence General population 4-7% (Caucasians) Cause of First DVT In 20% cases Thrombophilic families 40% incidence Genetic Basis Inheritance Mutation Autosomal dominant Exon 10 (G to A at position 1691) Physiologic Basis Derangement Basis Lack of inactivation of activated factor V Resistance of factor Va to cleavage by APC Risk for thrombosis Homozygous Heterozygous Relative risk of VTE increased to 80-fold Relative risk of VTE increased to 7-fold Laboratory testing Assay Functional assay for activated protein C PCR assay for factor VLeiden mutation detection PROTHROMBIN GENE MUTATION (G20210A): A specific mutation in the gene for prothrombin, which results in the replacement of Guanine (G) by Adenine (A) at position 20210 results in elevated levels of prothrombin. This is thought to create a hypercoagulable state by providing more substrate and hence more thrombin generation once the coagulation cascade is physiologically activated. It is speculated that subclinical physiologic activation of coagulation at local sites of minor injury in individuals with this mutation results in formation of unusually larger and/or frequent clot formation that would not have occurred in individuals without this mutation. ATTRIBUTES OF PROTHROMBIN GENE MUTATION (G20210A): Occurrence General population 1-2% heterozygosity (Caucasians) Cause of First DVT In 6% cases Thrombophilic families 18% incidence Genetic Basis Inheritance Mutation Autosomal dominant G to A at site 20210 in the 3`untranslated region Physiologic Basis Derangement Basis Increase thrombin formation Elevated prothrombin (factor II) in plasma The contents & pictures in this handout are derived from various sources including books, journal articles and patient material for teaching purposes only. No commercial incentives are sought or intended. Thrombotic Disorders 7 Risk for thrombosis Homozygous Heterozygous Increased risk of VTE & also for arterial thrombosis Relative risk of VTE increased to 3-fold Laboratory testing Assay PCR assay for prothrombin 20210 mutation PROTEIN C DEFICIENCY: As we learned previously that under physiologic conditions, protein C is converted to its activated form (APC) by thrombin:thrombomodulin complex on the endothelial cell surface after which APC inactivates factors Va and VIIIa. Lack of protein C function could be result of a quantitative or a qualitative defect. Type 1: Quantitative defect; (1a) Partial deficiency, (1b) Total deficiency Type 2: Qualitative defects ATTRIBUTES OF PROTEIN C DEFICIENCY: Occurrence General population 0.2% heterozygous (Caucasians) Cause of First DVT In 3% cases Thrombophilic families 6% incidence Genetic Basis Inheritance Mutation Autosomal recessive Many (>150 mutation) Physiologic Basis Derangement Basis Unregulated coagulation, decreased fibrinolysis Decreased inactivation of Va and VIIIa Risk for thrombosis Homozygous Heterozygous Neonatal purpura fulminans, cerebral vein thrombus Relative risk of VTE increased to 7-fold Laboratory testing Assay Functional assay detecting decreased anticoagulant activity or antigen level PROTEIN S DEFICIENCY: Protein S serves as a cofactor for protein C in the cleavage of factor Va and VIIIa and hence its deficiency either quantitative or qualitative also predisposes for thrombosis. Type 1 Type 2 Type 3 Protein S antigen Protein S activity Total Free Low Low Low Normal Normal Low Normal Low Low The contents & pictures in this handout are derived from various sources including books, journal articles and patient material for teaching purposes only. No commercial incentives are sought or intended. Thrombotic Disorders 8 ATTRIBUTES OF PROTEIN S DEFICIENCY: Occurrence General population Uncertain Cause of First DVT In 1-2% cases Thrombophilic families 6% incidence Genetic Basis Inheritance Mutation Autosomal dominant Many (>150 mutation) Physiologic Basis Derangement Basis Reduced protein C function Decreased cofactor activity for protein C Risk for thrombosis Homozygous Heterozygous Neonatal purpura fulminans, cerebral vein thrombus Relative risk of VTE increased to 6-fold Laboratory testing Assay Functional assay, decreased “free” protein S activity ANTITHROMBIN III DEFICIENCY: Antithrombin III (ATIII) is a major inhibitor of proteinase coagulation factors. Type 1:Quantitative defect Type 2:Qualitative defect (Thrombin binding site defects) Type 3:Qualitative defect (Heparin binding site defects) ATTRIBUTES OF ANTITHROMBIN III DEFICIENCY: Occurrence General population 0.02% Cause of First DVT In ~1% cases Thrombophilic families 4% incidence Genetic Basis Inheritance Mutation Autosomal dominant Many (~200 mutation) Physiologic Basis Derangement Basis Reduced antithrombin III activity Reduced inhibition of thrombin & other proteinases Risk for thrombosis Homozygous Heterozygous Laboratory testing Assay Lethal in utero Relative risk of VTE increased to 5-fold Functional assay for decreased activity The contents & pictures in this handout are derived from various sources including books, journal articles and patient material for teaching purposes only. No commercial incentives are sought or intended. Thrombotic Disorders 9 ACQUIRED RISK FACTORS OF THROMBOPHILIA ANTIPHOSPHOLIPID SYNDROME (APLS): Antiphospholipid syndrome is a clinicopathologic entity characterized by both venous and arterial thromboembolism, including thrombotic strokes, and recurrent fetal loss, and prolongation of in vitro phospholipid based coagulation tests such as aPTT, PT, and dilute Russell Viper Venom Time (dRVVT). It is perhaps the most common acquired risk factor for unprovoked thrombosis. Antiphospholipid antibodies (APLA) include a broad family of autoantibodies that include (a) Lupus anticoagulant (LA) – so named because it was first identified in patients with systemic lupus erythromatosus (SLE); the term anticoagulant is a misnomer because they cause thrombosis instead of bleeding. (b) Anticardiolipin antibodies (ACA) – so named because it was first shown to react with cadiolipin from bovine heart in the test reagent. These APLAs are immunoglobulins (IgG, IgM, IgA and mixtures) that occur in <5% of normal adult individuals, in a proportion of patients with certain infections and with a higher frequency in patients with autoimmune disorders. FEATURES OF ANTIPHOSPHOLIPID SYNDROME: A) Clinical features: 1. Venous and arterial thromboembolism 2. Unexplained pregnancy losses 3. Thrombocytopenia 4. Stroke 5. Coronary artery disease 6. Livedo reticularis skin lesions, necrotizing skin vasculitis 7. Microangiopathy 8. Other features B) Laboratory criteria: either 1 or 2 or both 1. Anticardiolipin antibodies identified on two or more occasions at least 6 week apart as measured by enzyme linked immunosorbant assay (ELISA) 2. Lupus anticoagulant antibodies detected on two or more occasions at least 6 week apart in the following manner (a) Prolongation of aPTT, PT, kaolin clotting time, dRVVT (b) Failure to correct the prolonged coagulation time by mixing studies (c) Correction of coagulation time by the addition of excess phospholipid in the test reagent (d) Exclusion of other coagulopathies The contents & pictures in this handout are derived from various sources including books, journal articles and patient material for teaching purposes only. No commercial incentives are sought or intended. Thrombotic Disorders 10 THROMBOPHILIC PREDISPOSITION: There are many hereditary predispositions, any one or several of which can be complicated by an acquired illness, the combination of which could amplify the thrombotic risk. The thrombotic risk of a given hereditary predisposition or an acquired illness varies considerably, such that a low-risk condition alone is unlikely to cause a clinical venous thromboembolic disease (VTED). A combination of two genetic factors or a genetic factor plus an acquired factor could amplify the risk to a modest or high level. Some hereditary and acquired disorders carry more risk than others. RISK Low HEREDITARY ACQUIRED Heterozygous factor VLeiden General surgery Heterozygous prothrombin 20210 Oral contraceptive/Pregnancy mutation Immobilization Sickle cell anemia Elevated factor VIII levels Moderate Heterozygous protein C deficiency Heterozygous protein S deficiency Heterozygous AT-III deficiency High Homozygous factor VLeiden Surgery for total hip or Homozygous prothrombin 20210 replacement mutation Hip fracture Acute promyelocytic leukemia Surgery for malignancy Sepsis Antiphospholipid syndromes Myeloproliferative syndromes Paroxysmal nocturnal hemoglobinuria knee Excessive Homozygous protein C deficiency Mucin secreting adenocarcinomas Homozygous protein S deficiency Homozygous AT-III deficiency Double heterozygous for protein C, protein S and AT-III deficiency. Heterozygosity for factor VLeiden mutation + heterozygosity for protein C, S or AT-III deficiency The contents & pictures in this handout are derived from various sources including books, journal articles and patient material for teaching purposes only. No commercial incentives are sought or intended. Thrombotic Disorders 11 LABORATORY TESTING: In a patient with clinical diagnosis of thrombosis, following approach may be taken. In addition prothrombin gene mutation (G20210A) and fibrinogen levels are also recommended. Laboratory testing is best performed several weeks after completion of a course of oral anticoagulants in patients with thrombosis, to avoid confounding effects of acute thrombosis or heparin or warfarin therapy on the assay results. With the exception of protein C and protein S assays, all other testing can be performed in patients taking oral anticoagulants. [This picture is adapted from Dacie & Lewis Practical Haematology by Lewis et al. 9th ed. Churchill-Livingstone] FBC MSU FOB CXR PNH MPD VTE APCR FVL LAC ACLA AT FVIII Lp (a) TG HDL LDL Full blood count (complete blood count, CBC) Midstream urine specimen for evaluation of nephritic syndrome Fecal occult blood for gastrointestinal cancers Chest X-ray for lung cancers Paroxysmal nocturnal hemoglobinuria Myeloproliferative disorders Venous thromboembolism Activated protein C resistance assay Fcator V Leiden assay Lupus anticoagulant Anticardiolipin assay Antithrombin III Factor VIII assay Lipoprotein little a Triglycerides High density lipoprotein Low density lipoprotein The contents & pictures in this handout are derived from various sources including books, journal articles and patient material for teaching purposes only. No commercial incentives are sought or intended. Thrombotic Disorders 12 INDICATIONS FOR LABORATORY TESTINGS: A laboratory testing for thrombophilia should be performed if the results of testing could make a difference in the clinical care of the patient or family members. These conditions include: Changes in the duration or intensity of oral anticoagulation Administration of specific therapy (antithrombin concentrates, vitamins for homocyteinemia) More intense prophylaxis for high-risk situations (e.g. surgery, acute illness) Prolonged immobilization (transcontinental flights) with a family history of thrombosis Better accuracy in estimates of the future risk of thrombosis in clinical settings (surgery and pregnancy) Counseling of women as to the risks of oral contraceptives, pregnancy or hormone replacement therapy Study of family members at risk of thrombosis FUNCTIONAL ASSAYS: (a) Activated protein C resistance (b) Protein C levels (c) Protein S levels (d) Antithrombin III levels (e) Factor VIII levels PLASMA LEVELS: (a) Plasma homocystein levels (b) Plasma fibrinogen levels MOLECULAR TESTS (POLYMERASE CHAIN REACTION): (a) Factor VLeiden mutation (b) Prothrombin gene mutation (G20210A) The contents & pictures in this handout are derived from various sources including books, journal articles and patient material for teaching purposes only. No commercial incentives are sought or intended.