SOP example
advertisement

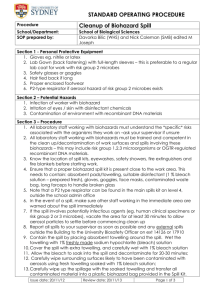
STANDARD OPERATING PROCEDURE SOP No: School/Department: Supervisor/Manager: Process School of Biological Sciences GMO Spill procedure in a PC2 laboratory Section 1 - Procedure 1. All laboratory staff must be trained and be competent in the clean up/decontamination of work surfaces and spills in a PC2 laboratory; 2. GMOs spills must be reported to your supervisor immediately. Make sure other staff working in the immediate area are warned about the spill; 3. All spills are to be assumed to contain potentially infectious material and the area must be vacated for at least 30 minutes to allow any aerosol particles to settle; 4. Report all spills to your supervisor and any external spills outside the PC2 laboratory to Jenny Dawkins (Biosafety Officer, OHSIM, K07), ext 14126 Section 2 – Potential Hazards 1. Infection; 2. Irritation; 3. Eye splash Section 3 – Personal Protective Equipment 1. Gloves; 2. Lab Gown (back fastening) with full-length sleeves; 3. Safety glasses or goggles; 4. Face mask or respirator classified P1(for Risk group 1 microorganisms) or P2 (Risk group 2 microorganisms) 5. PC2 spill kit (containing absorbent pads/towelling, disinfectant, gloves, goggles, face masks, contaminated waste bag, long forceps if broken glass involved etc) Section 4 – Handling, Storage and Disposal Requirements 1. Ensure contaminated objects and materials are placed into the biohazard bag for appropriate decontamination or hazardous waste disposal; Section 5 – Spill and Accident Procedure 1. Contain the spill with absorbent towels placed around the spill; 2. Soak extra absorbent towelling with freshly made sodium hypochlorite (bleach) solution (1.0% available chlorine: for a 4% bleach stock take 25 mls of bleach stock and dilute to 100ml); 3. Cover the spill with this towelling and allow the bleach to soak into the spill and decontaminate for 20-30 minutes; 4. Then carefully wipe surrounding surfaces likely to have been contaminated with aerosols using towelling soaked with bleach solution; 5. Carefully wipe up the spillage with the soaked towelling and transfer all contaminated material into a plastic biohazard bag provided in the Spill Kit. Section 6 – Decontamination Procedures 1. The generated waste and non-disposable contaminated gowns (if any) should be autoclaved in accordance with the GMO waste disposal SOP. Warning: Hypochlorite solutions should not be autoclaved. 2. Remove gloves, gown, safety glasses and face mask/respirator and wash hands thoroughly. Section 7 - Material safety data sheets (to be available and accessible) 1. Sodium hypochlorite; Chlorine; 2. Microorganisms/GMOs involved Section 8 - References 1. OHSIM (www.usyd.edu.au/ohs) website; 2. AS/NZS 2243.1:2005 – Safety in Laboratories: Planning and Operational Aspects; 3. AS/NZS 2243.3:2002 – Safety in Laboratories: Microbiological aspects and containment facilities; 4. AS/NZS 2243.10:2004 – Safety in Laboratories: Storage of Chemicals; 5. AS/NZS 2647:2000 – Biological safety cabinets: Installation and use. 6. OGTR Guidelines for Certification of a Physical Containment Level 2 Laboratory (Version 3.1) http://www.ogtr.gov.au/rtf/certification/PC2LABv3-1-1.rtf Issue date: Review date: Page 1 of 2 OHS Consultation and Approval (Ensure this section is completed and copied onto rear of SOP) (Completion Instructions) Print names and enter signatures and dates to certify that the persons named in this section have been consulted/trained in relation to the development and implementation of this Standard Operating Procedure. Note that the OHS Representative (OHS Committee) certifies that consultation has taken place, and may not be involved in the original consultation. Position Name Signature Date Manager/ Supervisor First employee using SOP Second employee using SOP (if applicable) Third employee using SOP (if applicable) OHS Representative (OHS Committee) SOP Approval Name Authorising (Printed): ..................................................................................................... Signature: ..........................................................................Date: ................................................ Issue date: Review date: Page 2 of 2