Biohazard Spill - The University of Sydney
advertisement
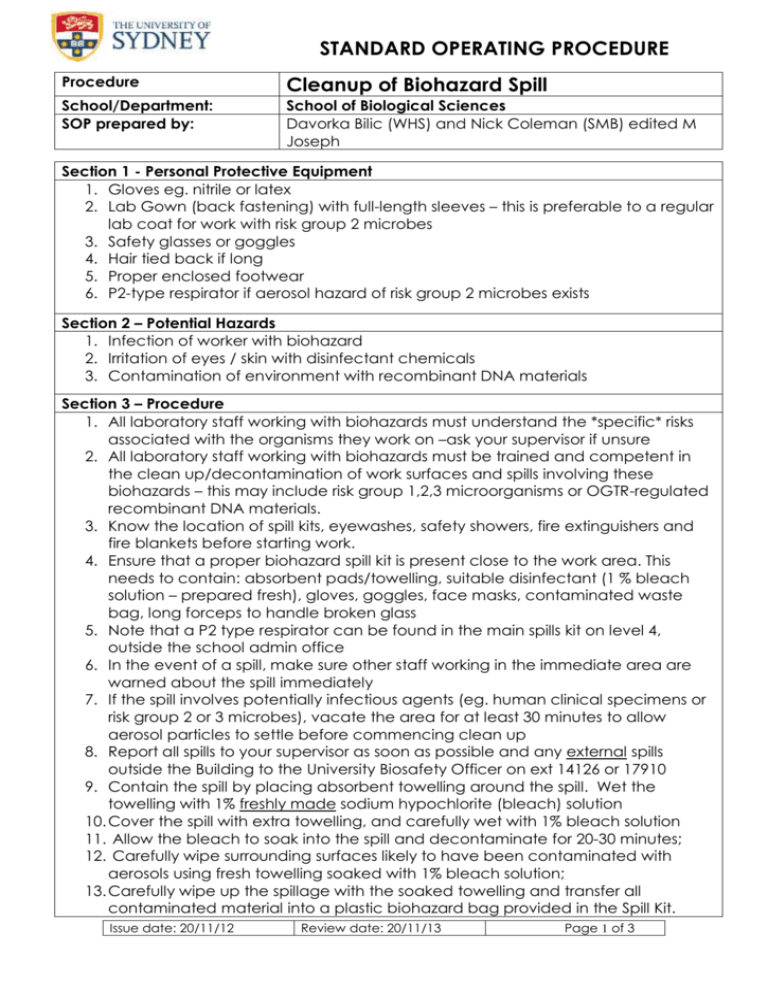
STANDARD OPERATING PROCEDURE Procedure Cleanup of Biohazard Spill School/Department: SOP prepared by: School of Biological Sciences Davorka Bilic (WHS) and Nick Coleman (SMB) edited M Joseph Section 1 - Personal Protective Equipment 1. Gloves eg. nitrile or latex 2. Lab Gown (back fastening) with full-length sleeves – this is preferable to a regular lab coat for work with risk group 2 microbes 3. Safety glasses or goggles 4. Hair tied back if long 5. Proper enclosed footwear 6. P2-type respirator if aerosol hazard of risk group 2 microbes exists Section 2 – Potential Hazards 1. Infection of worker with biohazard 2. Irritation of eyes / skin with disinfectant chemicals 3. Contamination of environment with recombinant DNA materials Section 3 – Procedure 1. All laboratory staff working with biohazards must understand the *specific* risks associated with the organisms they work on –ask your supervisor if unsure 2. All laboratory staff working with biohazards must be trained and competent in the clean up/decontamination of work surfaces and spills involving these biohazards – this may include risk group 1,2,3 microorganisms or OGTR-regulated recombinant DNA materials. 3. Know the location of spill kits, eyewashes, safety showers, fire extinguishers and fire blankets before starting work. 4. Ensure that a proper biohazard spill kit is present close to the work area. This needs to contain: absorbent pads/towelling, suitable disinfectant (1 % bleach solution – prepared fresh), gloves, goggles, face masks, contaminated waste bag, long forceps to handle broken glass 5. Note that a P2 type respirator can be found in the main spills kit on level 4, outside the school admin office 6. In the event of a spill, make sure other staff working in the immediate area are warned about the spill immediately 7. If the spill involves potentially infectious agents (eg. human clinical specimens or risk group 2 or 3 microbes), vacate the area for at least 30 minutes to allow aerosol particles to settle before commencing clean up 8. Report all spills to your supervisor as soon as possible and any external spills outside the Building to the University Biosafety Officer on ext 14126 or 17910 9. Contain the spill by placing absorbent towelling around the spill. Wet the towelling with 1% freshly made sodium hypochlorite (bleach) solution 10. Cover the spill with extra towelling, and carefully wet with 1% bleach solution 11. Allow the bleach to soak into the spill and decontaminate for 20-30 minutes; 12. Carefully wipe surrounding surfaces likely to have been contaminated with aerosols using fresh towelling soaked with 1% bleach solution; 13. Carefully wipe up the spillage with the soaked towelling and transfer all contaminated material into a plastic biohazard bag provided in the Spill Kit. Issue date: 20/11/12 Review date: 20/11/13 Page 1 of 3 14. Dry area with clean towelling. Dispose of this towelling also into the same biohazard bag. 15. The bleach-containing waste should be labelled as “Bleach-containing spill cleanup” and the name / type of the biohazard, then disposed of in the large yellow ‘Sulo’ bin Behind Macleay Building A12 – do not autoclave bleachcontaining materials ! 16. Remove gloves, gown, safety glasses and face mask/respirator and wash hands thoroughly. If lab gown has been contaminated, autoclave this ASAP. Section 4 - Material safety data sheets (to be available and accessible) 1. Sodium hypochlorite; Chlorine; 2. Microorganisms/GMOs involved Section 5 - References 1. WHSS (sydney.edu.au/whs/guidelines/biosafety/index.shtml) website; 2. AS/NZS 2243.1:2005 – Safety in Laboratories: Planning and Operational Aspects; 3. AS/NZS 2243.3:2010 – Safety in Laboratories: Microbiological safety and containment; 4. AS/NZS 2243.10:2004 – Safety in Laboratories: Storage of Chemicals; 5. AS/NZS 2252.4:2010 – Biological safety cabinets Classes I & II - Installation and use. 6. OGTR Guidelines for Certification of a Physical Containment Level 2 Laboratory Risk assessment for biohazard spills 7. Risk assessment and SOP for Risk group 2 microorganisms and/or Animals+Animal Tissues and/or Humans/human tissues Issue date: 20/11/12 Review date: 20/11/13 Page 2 of 3 SOP Training Confirmation By signing below, these individuals confirm that they have read and understood the SOP, and agree to always follow the instructions in this SOP when performing this procedure. Position Name Signature Date Supervisor employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student employee / student WHS Committee Approval Representative: A. Prof Frank Seebacher Chair Safety Committee Signature: ........................................................ Date: ..................................... Issue date: 20/11/12 Review date: 20/11/13 Page 3 of 3