lasers lab - group6-pchem-gsu
advertisement
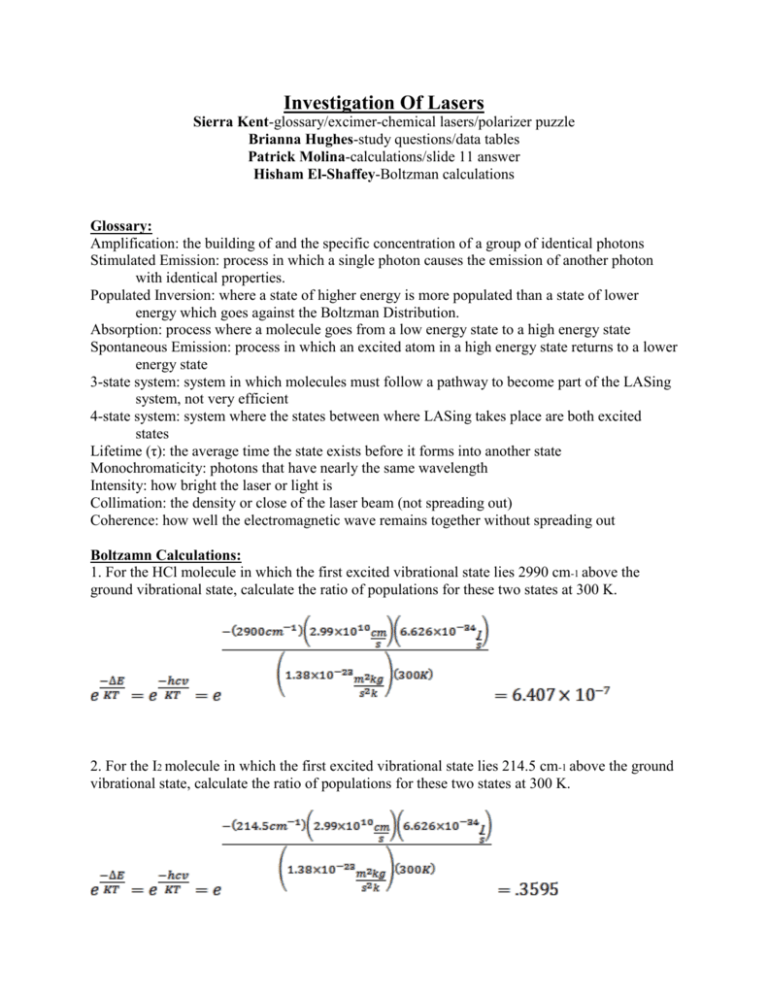
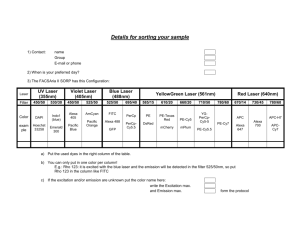
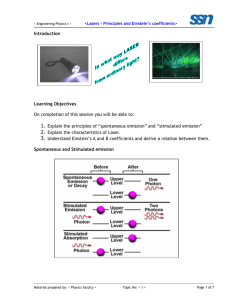
Investigation Of Lasers Sierra Kent-glossary/excimer-chemical lasers/polarizer puzzle Brianna Hughes-study questions/data tables Patrick Molina-calculations/slide 11 answer Hisham El-Shaffey-Boltzman calculations Glossary: Amplification: the building of and the specific concentration of a group of identical photons Stimulated Emission: process in which a single photon causes the emission of another photon with identical properties. Populated Inversion: where a state of higher energy is more populated than a state of lower energy which goes against the Boltzman Distribution. Absorption: process where a molecule goes from a low energy state to a high energy state Spontaneous Emission: process in which an excited atom in a high energy state returns to a lower energy state 3-state system: system in which molecules must follow a pathway to become part of the LASing system, not very efficient 4-state system: system where the states between where LASing takes place are both excited states Lifetime (τ): the average time the state exists before it forms into another state Monochromaticity: photons that have nearly the same wavelength Intensity: how bright the laser or light is Collimation: the density or close of the laser beam (not spreading out) Coherence: how well the electromagnetic wave remains together without spreading out Boltzamn Calculations: 1. For the HCl molecule in which the first excited vibrational state lies 2990 cm-1 above the ground vibrational state, calculate the ratio of populations for these two states at 300 K. 2. For the I2 molecule in which the first excited vibrational state lies 214.5 cm-1 above the ground vibrational state, calculate the ratio of populations for these two states at 300 K. 3. At what temperature will the ratio for HCl equal the ratio for I2 found in example 2? Slide 11 Answer: A continuous oscillating input of energy would allow for the maintenance of the population inversion allowing for a continuous emission of light. Excimer and Chemical Lasers: An excimer laser is nearly always operated in the ultraviolet (UV) spectral region and generates nanosecond pulses. The laser is a gas mixture, typically containing a noble gas and a halogen. An excimer gain medium is pumped with short current pulses in a high-voltage electric discharge, which create excimers or excited dimers which are molecules represent only in the excited electronic state, but not in the electronic ground state, giving the population inversion needed giving the laser beam. A chemical laser is a laser that achieves its populated inversion from excited molecules that are created during a chemical reaction. A gas is injected into the reaction and reacts with those molecules to make excited molecules. These excited molecules then form a population inversion, and undergo stimulated emission, producing a powerful laser beam. Data and Calculations: For first He/Ne laser m1 m2 θ1 θ2 -1 d for θ1 d-1 for θ2 Values 246.1 cm 84.52 cm 22.09° 49.78° 6.033x105 grating lines/mm 5.94x105 grating lines/mm For second He/Ne laser using meter stick m1 m2 θ1 θ2 -1 d for θ1 d-1 for θ2 Values 87 cm 67 cm 22.52° 46.31° 5 6.053x10 grating lines/mm 5.713x105 grating lines/mm Calculations a=1m b=0.8452m c=1.309m m2=0.8452 mλ=dsinθ 2(632.8nm)/(sin(49.78)) = d 6.033x105=d-1 Polarization puzzle: The argon laser had two polarizers placed in front of its beam. One was polarized to make all light face the same direction perpendicular to the beam itself and the second was polarized to make the light face perpendicular to the first polarizer theoretically canceling out all light. Since our polarizers were not perfect there was a dim light instead of no light. We then placed another polarizer which was held in between the two polarizers and rotated. When rotated the light dimmed and brightened which occurred because the polarization was not set to let only one direction of light through. The light dimmed when the polarizer was turned allowing the opposite direction of light coming from the first polarizer, which was cancelled. The light got brighter if the third polarizer was turned in a direction that was the same as the light from the first polarizer allowing it to pass through. Study Questions: 1. How are photons created? When energy is released from the collision of electrons a photon is created. 2. How is amplification accomplished? By spontaneous emission; having more excited atoms than in the ground state. 3. What population is inverted? Energy states, there are more in higher states. 4. Why must a population be inverted? In order that lasers can occur. 5. What does absorption have to do with the process? Competes with stimulated emission. If this process overcomes stimulated emission then no laser occurs. 6. What does pumping mean AND why is it important? Exciting atoms for stimulated emission in production and amplification of laser signals. It’s used to stimulate emission. Important to create inversion necessary for laser action to take place. 7. How does stimulated emission compare to absorption? It is opposite. Goes from a higher state to a lower state. 8. What is the difference between stimulated emission and spontaneous emission? Spontaneous does not require the stimulation of a photon, but instead gives off a photon by transforming to a state of lower energy. Stimulated is where one photon causes emission of another photon of identical properties.