Set1
advertisement
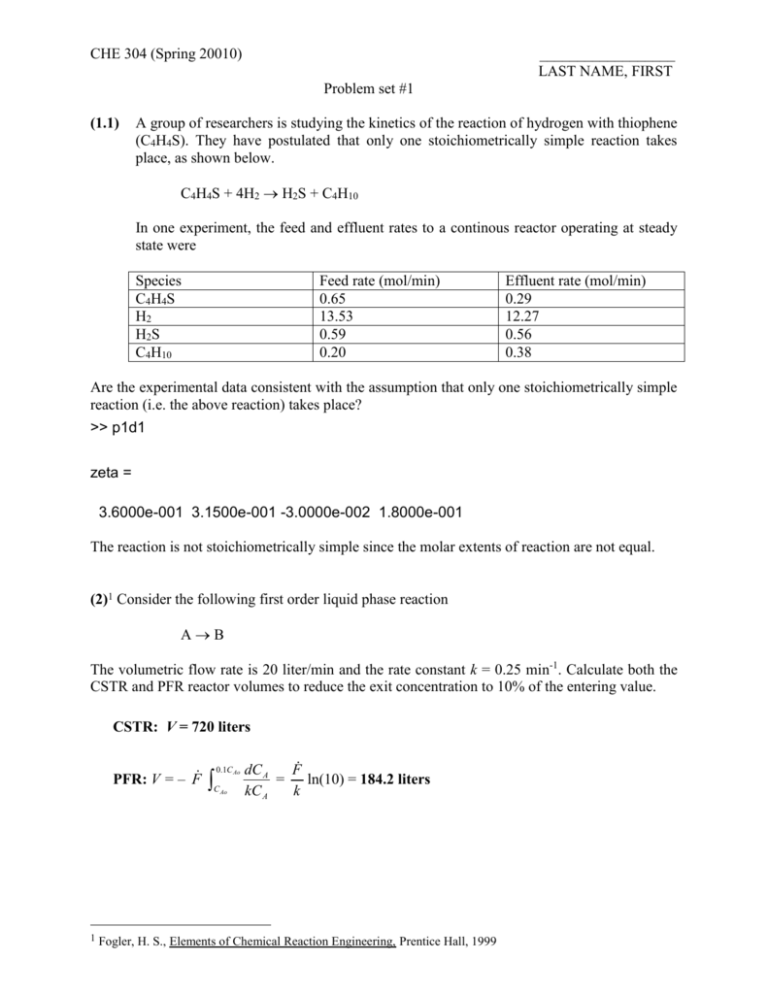
CHE 304 (Spring 20010) __________________ LAST NAME, FIRST Problem set #1 (1.1) A group of researchers is studying the kinetics of the reaction of hydrogen with thiophene (C4H4S). They have postulated that only one stoichiometrically simple reaction takes place, as shown below. C4H4S + 4H2 H2S + C4H10 In one experiment, the feed and effluent rates to a continous reactor operating at steady state were Species C4H4S H2 H2S C4H10 Feed rate (mol/min) 0.65 13.53 0.59 0.20 Effluent rate (mol/min) 0.29 12.27 0.56 0.38 Are the experimental data consistent with the assumption that only one stoichiometrically simple reaction (i.e. the above reaction) takes place? >> p1d1 zeta = 3.6000e-001 3.1500e-001 -3.0000e-002 1.8000e-001 The reaction is not stoichiometrically simple since the molar extents of reaction are not equal. (2)1 Consider the following first order liquid phase reaction AB The volumetric flow rate is 20 liter/min and the rate constant k = 0.25 min-1. Calculate both the CSTR and PFR reactor volumes to reduce the exit concentration to 10% of the entering value. CSTR: V = 720 liters PFR: V = – F 1 0.1C Ao C Ao dC A F = ln(10) = 184.2 liters kC A k Fogler, H. S., Elements of Chemical Reaction Engineering, Prentice Hall, 1999 (3)1 Consider the following first order liquid phase reaction AB The rate constant, k, is equal to 0.20 min-1. Calculate the time to reduce the number of moles of A to 2% of its initial value in a constant volume batch reactor. t = 19.56 min (4)1 Consider the following first order liquid phase reaction AB Calculate both the CSTR and PFR reactor volumes to reduce the exit concentration to 1% of the entering value. The entering molar flow rate is 5 mol/hr and the entering volumetric flow rate is 10 L/hr. The following reaction rate is assumed: (a) rA = k with k = 0.05 mol/Lhr (b) rA = kCA with k = 0.0001 s-1 (c) rA = kCA2 with k = 3 L/molhr CSTR (a) rA = k V = 0.99 FC A0 (0.99)(10)(0.5) = = 99 liters k 0.05 (b) rA = kCA V = (c) rA = kCA2 V = 0.99 F 0.99 FC A0 0.99(10) = = = 2750 liters 0.01k 0.01kC A0 0.01(0.0001)(3600) 0.99 FC A0 0.012 k C A0 2 = 0.99 F 0.99(10) = = 66,000 liters 2 0.01 kC A0 0.012 (3)(0.5 PFR (a) rA = k V = F C A0 C A 0.99 FC A0 (0.99)(10)(0.5) = = = 99 liters k 0.05 k (b) rA = kCA V = F C A0 10 ln ln(100) = 128 liters = k C A 0.0001(3600) (c) rA = kCA2 V = 1 10 1 F 1 = 3 0.5 (100 1) = 660 liters k C A C A0 (5)1 The gas phase reaction AB+C is carried out isothermally in a 25 L constant-volume batch reactor. Twenty moles of pure A is initially placed in the reactor. The reactor is well mixed. (a) If the reaction is first order: rA = kCA with k = 0.865 min-1 calculate the time necessary to reduce the number of moles of A in the reactor to 0.2 mol. (b) If the reaction is second order: rA = kCA2 with k = 2 L/molmin calculate the time necessary to consume 19 moles of A. (c) If the temperature is 127oC, what is the initial total pressure? What is the final total pressure assuming the reaction goes to completion? (a) t = 9.21 min (b) t = 47.5 min (c) P = 26.24 atm R = 0.082 Latm/moloK Final conditions: n = 40 moles P = 52.48 atm (6)1 The gas phase reaction A(g) + B(g) = C(g) was studied at 100 and 200oC and at a pressure of 1 atm. The following equilibrium mol fractions were obtained: yC yA yB 100oC 0.172 0.414 0.414 200oC 0.642 0.179 0.179 If equimolar quantities of A and B are reacted at 150oC and a pressure of 10 atm, what will be the equilibrium composition? yC = /(2 ) = 0.761; yA = yB = 0.11946 (7)1 The chemical species A is known to decompose according to A(g) = B(g) + C(g) A rigid container is filled with pure gaseous A at 300oK and 760 mmHg and then heated. The pressure was observed to be 1114 mmHg at 400oK and 1584 mmHg at 500oK. Estimate the pressure for a temperature of 600oK. Assume ideal-gas behavior and chemical equilibrium. P = 1520(1 + ) = 2166 mmHg (8) Run Staging (http://www.engin.umich.edu/~cre/icm/cre.html) You will find the program Staging in the CHE 304 distribution folder, then ICM, then Staging, then click on Staging.exe. Turn in the last page of the program with performance number. 1 Kyle, B.G., Chemical and Process Thermodynamics, Prentice Hall, 1999