Course 323
Course Objectives
أهداف المقرر
مقرر عملي يهدف إلعطاء الطالب أساسيات في تحضير مترا كبات العناصر االنتقالية
The course places a strong and rigorous emphasis on good
laboratory notebook skills, professionally written lab notes, and
oral communication skills. Critical thinking is the backbone of the
course; students should report their results with analysis and
conclusions.
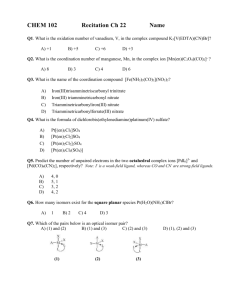
The successful student will gain an understanding into:1) Inorganic synthesis.
2) Metal-ligand interaction and reactivity
3) Physical characterization of inorganic substances via the
application of classical and modern methods of analysis, including
the use of modern instrumentation (IR, UV/ Vis, etc...) leading to
structural prediction.
Learning Resources
1
2
3
4
5
References:Introduction to Semi micro Qualitative Analysis, Eighth Edition.
J.J. Lagowski and C.H. Sorum. Pearson Prentice Hall. 2004.1st
edition (Ask your instructor).
Vogel’s Qualitative Inorganic Analysis, sixth edition. G Svehla,
Longman Scientific and Technical. 1990.6th (Ask your instructor,
Main library, Science library).
General Chemistry with Qualitative Analysis, 5th edition. Whitten,
Davis, Peck. Harcourt Brace College Publishing. 2004 (Main
library).
http://www.wcrl.ars.usda.gov/cec/java/convert8.htm
www.school-for- champions.com/science/chemixture_types.htm
Lab requirements and Safety precautions :-
1. Calculator with scientific functions.
2. Bound laboratory notebook for recording data and observations.
3. Approved safety glasses should always be worn in a laboratory
environment.
4. Laboratory coat (to be discussed during the first scheduled
laboratory period)
5. Proper footwear (to be discussed during the first scheduled
laboratory period).
Admittance to the laboratory will be permitted only if the student
has all items for each lab.
Student assessment:
Student assessment:
Mid Term 20
Final
20
Lab work 60
Total
100
Important rules of academic conduct
Laboratory Rules:-
1)
2)
3)
4)
5)
Follow good laboratory techniques (ask your Lab instructor).
Protect yourself from accidents when using equipments.
Do not taste / inhale chemicals or place chemicals in your hand.
Do not set on the laboratory bench.
Take a position in the lab that you are protecting yourself as
well as others.
6) Use your own observations and analysis.
7) When studying chemical reaction it’s important to note: The reagent and any special experimental circumstances that
were applied when the test was performed.
The changes observed.
An equation that represents the reaction occurring.
Conclusion derived from the test.
Conclusions derived from UV/Vis and IR spectra.
Conclusions derived from metal and ligand analysis.
Student responsibilities to the course:1) Attendance.
2) Ability to answer questions being asked during the lecture.
3) Understand both theoretical and experimental part of the course.
4) Submit analysis and report on time.
5) Good notes recording is important.
Table of Contents
1. Preparation of [Cu(NH3)4]SO4.H2O
2. Preparation of [Cu(NH2CSNH2)3]1/2SO4.H2O
3. Preparation of [Co(NH3)6]Cl3
4. Preparation of [Al(acac)3]Cl3
5. Preparation of [Co(NH3)4CO3]NO3
6. Preparation of [Co(NH3)5Cl]Cl3
7. Preparation of [Cu(acac)2]
8. Preparation of [Fe(C2O4)2]and K3[Fe(C2O4)3]3H2O
9. Preparation of [Fe(acac)3]Cl3
10.Preparation of K[Cr(C2O4)2(H2O)2]
11.Preparation of [Ni(en)3]Cl2
12.Preparation of [Co(NO2)2(en)2]NO3
13.Preparation of [Ni(NH4)2(SO4)2.6H2O]
14.Preparation of [Ni(NH3)6]Cl3
15.Preparation of [Co(en)3]+3