Advanced Inorganic Chemistry 4305
advertisement

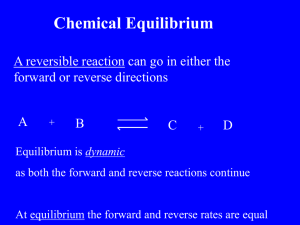
Advanced Inorganic Chemistry 4305 Homework assignment # 5 Chapters 11, 12 1. Predict whether these complexes would be labile or inert and explain (briefly) your choices. a) Ammonium oxopentachlorochromate(V) 1.83 μB b) Potassium hexaiodomanganate 3.82 μB c) Potassium hexacyanoferrate(III) 2.40 μB d) Hexammineiron(II) chloride 5.45 μB 2. The rate of H2O exchange by [M(H2O)6] is greater for M=Fe than for M=Ru by a factor of 108. Explain 3. Which of the following would have a greater rate constant for ligand exchange via an Id mechanism. Briefly explain your answer. a. CoCl3(PEt3)3 or CoCl3(PMe3)3 b. CoCl6 or CoCl3(CO)3 c. CoCl3(NH3)3 or CoCl3(H2O)3 d. CoCl6-3 or TiCl6-3 4. Data for the reaction: a. Co(NO)(CO)3 + As(C6H5)3 Co(NO)(CO)2(As(C6H5)3) + CO In toluene at 45oC is given below. Find the rate constant(s) for the substitution reaction [As(C6H5)3] (Mo/L) 0.0014 0.098 0.525 1.02 5. The rate constants for the reaction: kobs (s-1) 2.3 3.9 12 23 CrX+2 + *Cr+2 *CrX2+ + Cr2+ are: Xk (M-1 s-1) F 1.2 x 10-3 Cl 11 Br 60 N3 1.2 Explain the differences in the rate constants in terms of the probable mechanism of the reaction. 6. Write the platinum-containing product for each of the following reactions and indicate the stereochemistry. a. [Pt(NH3)Cl3]- + NO2- b. [Pt(CN)Cl3]- + NO2- c. [PtCl4]2- + 2 CO 7. When two possible isomers of [Pt(NH3)2Cl2] react with thiourea (tu) , one product is [Pt(tu)4]2+ and the other is [Pt(NH3)2 (tu)2]2+ . Identify the initial isomers and explain the results. 8. Suggest a structure for Cp2Fe(CO)2