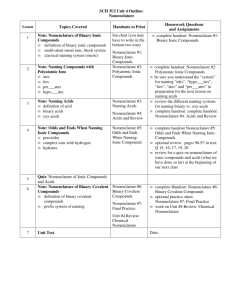
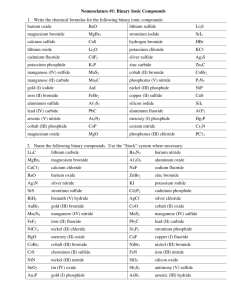
Homework Solutions
advertisement
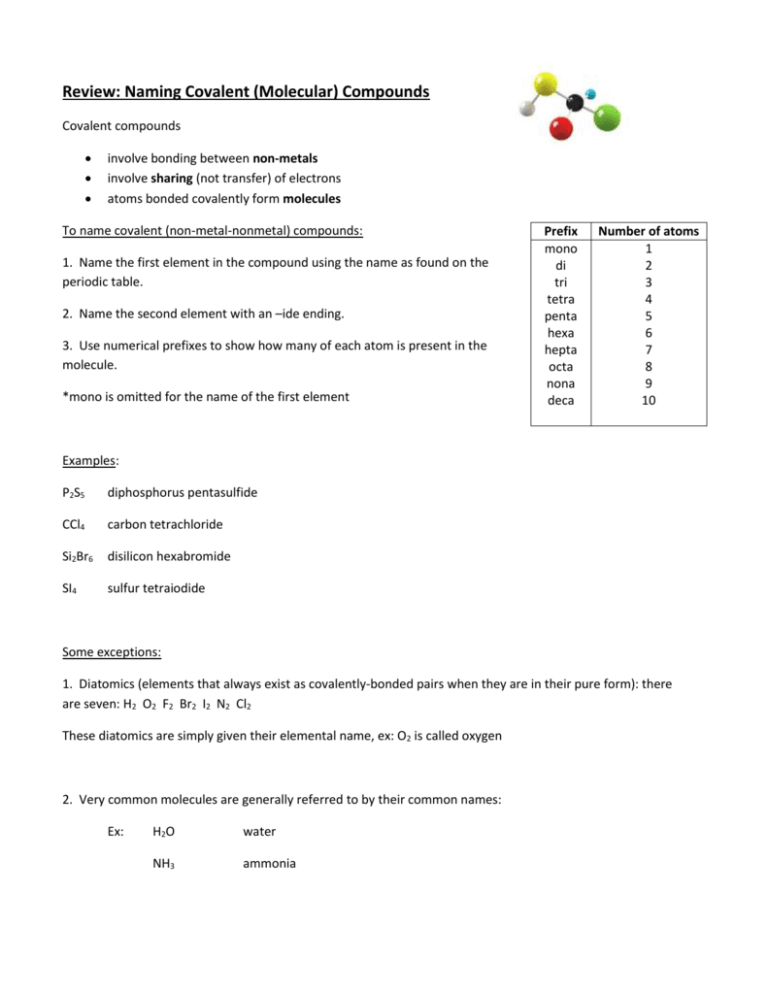
Review: Naming Covalent (Molecular) Compounds Covalent compounds involve bonding between non-metals involve sharing (not transfer) of electrons atoms bonded covalently form molecules To name covalent (non-metal-nonmetal) compounds: 1. Name the first element in the compound using the name as found on the periodic table. 2. Name the second element with an –ide ending. 3. Use numerical prefixes to show how many of each atom is present in the molecule. *mono is omitted for the name of the first element Prefix mono di tri tetra penta hexa hepta octa nona deca Number of atoms 1 2 3 4 5 6 7 8 9 10 Examples: P2S5 diphosphorus pentasulfide CCl4 carbon tetrachloride Si2Br6 disilicon hexabromide SI4 sulfur tetraiodide Some exceptions: 1. Diatomics (elements that always exist as covalently-bonded pairs when they are in their pure form): there are seven: H2 O2 F2 Br2 I2 N2 Cl2 These diatomics are simply given their elemental name, ex: O2 is called oxygen 2. Very common molecules are generally referred to by their common names: Ex: H2O water NH3 ammonia Name the following compounds 1. SO2 sulfur dioxide 2. CO carbon monoxide 3. ClO2 chlorine dioxide 4. N2O dinitrogen monoxide 5. P2O5 diphosphorus pentoxide 6. CBr4 carbon tetrabromide 7. N2S5 dinitrogen pentasulfide 8. N2O4 dinitrogen tetraoxide 9. BF3 boron trifluoride 10. SiCl4 silicon tetrachloride 11. IF7 iodine heptafluoride 12. P4O10 tetraphosphorus decaoxide 13. PO3 phosphorus trioxide 14. BrCl3 bromine trichloride 15. P2O4 diphosphorus tetraoxide 16. NF4 nitrogen tetrafluoride 17. S4N4 tetrasulfur tetranitride 18. NF3 nitrogen trifluoride 19. NO2 nitrogen dioxide 20. Cl2O7 dichlorine heptaoxide Write the formulas for the following compounds: 1. carbon dioxide CO2 2. nitrogen monoxide NO 3. nitrogen dioxide NO2 4. sulfur trioxide SO3 5. tetraphosphorus decoxide P4O10 6. tetraphosphorus hexoxide P4O6 7. iodine monochloride ICl 8. dihydrogen monoxide H2O 9. nitrogen trichloride NCl3 10. carbon disulfide CS2 11. disilicon hexabromide Si2Br6 12. tetranitrogen tetrasulfide N4S4 13. carbon monoxide CO 14. dinitrogen pentoxide N2O5 15. water H2O 16. ammonia NH3 17. phosphorus pentachloride PCl5 18. carbon tetrachloride CCl4 19. diselenium dichloride Se2Cl2 20. dinitrogen tetroxide N2O4 Naming Acids Acids are a special group of substances, they are compounds of non-metals but they form ions in solution. Acids can be recognized by the fact that their chemical formula generally begins with H Because acids are a unique group, they have different naming rules: Acid Example Anion Clchloride NO2nitrite NO3nitrate HCl HNO2 HNO3 Anion ending How To Name it Name -ide hydro(stem)ic acid hydrochloric acid -ite (stem)ous acid nitrous acid -ate (stem)ic acid nitric acid **Two stem ‘exceptions’: S sulfur – the stem is ‘sulfur’ ex. sulfuric acid P phosphorus – the stem is ‘phosphor’ ex. phosphoric acid Examples: Use the rules to name the following: 1. HBr 2. HClO4 3. H2S hydrobromic acid perchloric acid hydrosulfuric acid Complete the table with the missing acid names or formulas. Formula 1. HNO3 2. H2SO4 3. H2SO3 4. H2C2H3O2 5. H3PO4 6. H2S 7. H2CO3 8. HClO 9. H2S 10. H2F Name nitric acid sulfuric acid sulfurous acid acetic acid phosphoric acid hydrosulfuric acid carbonic acid hypochlorous acid hydrosulfuric acid hydrofluoric acid So…let’s put this all together!! When naming or writing chemical formulas, first determine what type of compound you are dealing with: Ionic metal+non-metal/polyatomic Covalent: non-metal+non-metal Acid: formula begins with H Type of Chemical Bonds Classify the following compounds as ionic, covalent, or acids. 1. CaCl2 Ionic 11. MgO Ionic 2. CO2 Covalent 12. CuCl2 Ionic 3. H2SO4 Acid 13. HCl Acid 4. BaSO4 Ionic 14. KI Ionic 5. K2O Ionic 15. NaOH Ionic 6. NaF Ionic 16. NO2 7. Na2CO3 Ionic 17. AlPO4 Ionic 8. CH4 Covalent 18. FeCl3 Ionic 9. SO3 Covalent 19. P2O5 Covalent Ionic 20. N2O3 Covalent 10. LiBr Covalent Naming Practice (all types of compounds) Name the following compounds: 1. CaCO3 calcium carbonate 2. SCl6 sulfur hexachloride 3. PCl5 phosphorus pentachloride 4. CaS calcium sulfide 5. Cu2SO3 copper (I) sulphite 6. K3N potassium nitride 7. NH4NO3 ammonium nitrate 8. Al(OH)3 aluminum hydroxide 9. HNO3 (aq) nitric acid 10. HBr (aq) hydrobromic acid 11. N2O4 dinitrogen tetraoxide 12. O2 oxygen 13. Mg3N2 magnesium nitride 14. NF3 nitrogen trifluoride 15. NH3 ammonia 16. H2CO3 (aq) carbonic acid 17. CS2 carbon disulfide 18. H2S (aq) hydrosulfuric acid 19. AuCl3 gold (III) fluoride 20. KMnO4 potassium permanganate Write the formulas of the following compounds: 1. potassium nitrate KNO3 2. iron (III) chloride FeCl3 3. acetic acid HC2H3O2 4. nitrous acid HNO2 5. nitrogen monoxide NO 6. zinc phosphate Zn3(PO4)2 7. hydrochloric acid HCl 8. tin (IV) chloride SnCl4 9. magnesium hydride MgH2 10. nickel (II) chlorate Ni(ClO3)2 11. diphosphorus pentoxide P2O5 12. ferric hydroxide Fe(OH)3 13. phosphoric acid H3PO4 14. strontium phosphide Sr3P2 15. sulfur dioxide SO2 16. aluminum oxide Al2O3 17. zinc fluoride ZnF2 18. carbon dioxide CO2 19. mercury (II) sulfate HgSO4 20. sulphuric acid H2SO4