Solutions
advertisement

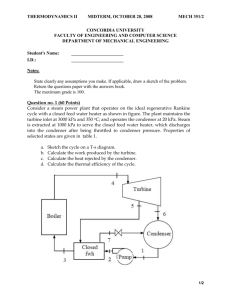
College of Engineering and Computer Science Mechanical Engineering Department Mechanical Engineering 370 Thermodynamics Fall 2010 Course Number: 14319 Instructor: Larry Caretto Unit Eleven Homework Solutions, November 30, 2010 1. Consider a 210 MW steam power plant that operates on a simple ideal Rankine cycle. Steam enters the turbine at 10 MPa and 500oC and is cooled in the condenser to a pressure of 10 kPa. Show the cycle on a T-s diagram with respect to the saturation lines and determine (a) the quality of steam at the turbine exit, (b) the thermal efficiency of the cycle, and (c) the mass flow rate of the steam. Rankine Cycle Diagram 900 3 800 Temperature (K) 700 600 500 400 2 300 1 4 200 1234- 100 2 Pum p 3 Steam generator 4 Turbine 1 Condenser 0 0 1 2 3 4 5 6 7 8 9 10 Entropy (kJ/kg-K) Saturation Condenser Pump Steam Generator Turbine The cycle diagram shows the individual steps in the cycle. The increase in temperature in the pump is typically about 1oC so the isentropic pump step does not really show on the diagram. The constant pressure heating in the steam generator shows the path of an isobar on a T-s diagram. In the mixed region, where temperature and pressure are constant, the isobar is a horizontal line. The condenser, which is completely in the mixed region, has a constant temperature line to represent the constant pressure process in the condenser. To compute the quality at the turbine exit, we recognize that this exit state is defined by the condenser pressure of 10 kPa and an isentropic process such that s out = sin = s(10 MPa, 500oC) = 6.5995 kJ/kg∙K. The outlet quality is thus found from the following equation. Jacaranda (Engineering) 3519 E-mail: lcaretto@csun.edu Mail Code 8348 Phone: 818.677.6448 Fax: 818.677.7062 Unit eleven homework solutions xout ME 370, L. S. Caretto, Fall 2010 s out s f (10 kPa) s fg (10 kPa) 6.5995 kJ kg K 0.6492 kJ 7.4996 kJ Page 2 kg K xout = 0.793 . kg K In order to compute the efficiency, we need the enthalpy values at all the state points. Following a conventional Rankine cycle calculation, we find the properties at state one as those of a saturated liquid at the condenser pressure: h1 = hf(10 kPa) = 191.81 kJ/kg and v1 = 0.001010 m3/kg. The isentropic pump work, |wp| = v1(P2 – P1) where P2 is the same as the inlet pressure to the turbine, 10 MPa = 10,000 kPa. Thus, wP1 v1 P2 P1 0.001010 m 3 10000 kPa 10 kPa 1 kJ 3 10.09 kJ kg kPa m kg We then find h2 = h1 + |wP1| = 191.81 kJ/kg + 10.09 kJ/kg = 201.90 kJ/kg. h3 = h(10 MPa, 500oC) = 3375.1 kJ/kg. s3 = s(10 MPa, 500oC) = 6.5995 kJ/kg∙K. As noted above, state 4 is in the mixed region with P4 = 10 kPa and x4 = 0.793. We thus find the enthalpy from the quality as h4 = hf(P4 = 10 kPa) + x4 hfg(P4 = 10 kPa) = 191.81 + (0.793)(2392.1 kJ/kg) or h4 = 2089.7 kJ/kg. The heat input to the steam generator, qh = h3 – h2 = 3375.1 kJ/kg – 201.90 kJ/kg = 3173.2 kJ/kg The condenser heat rejection, ql = |h1 – h4| = |191.81 kJ/kg – 2089.7 kJ/kg| = 1897.9 kJ/kg. The net work, w = qh - |qL| = 3173.2 kJ/kg| - 1897.9 kJ/kg = 1275.3 kJ/kg. The efficiency = w / qH = (1275.3 kJ/k ) / (3173.2 kJ/kg) or = 40.2% . The mass flow, m 210 MW 1000 kJ W w 1275.3 kJ MW s kg m 165 kg s 2. Consider a solar-pond power plant that operates on a simple ideal Rankine cycle with refrigerant-134a as the working fluid. The refrigerant enters the turbine as a saturated vapor at 1.6 MPa and leaves at 0.7 MPa. The mass flow rate of the refrigerant is 6 kg/s. Show the cycle on a T-s diagram with respect to the saturation lines and determine (a) the thermal efficiency and (b) the power output of the plant. The cycle diagram is shown on the next page. As usual, when the T-s diagram is drawn to scale, the pump does not appear on the diagram and the constant-pressure heating of the liquid in the steam generator is very close to the saturation line. This diagram is unusual because there is no superheating. In addition, the particular inlet and outlet pressures chosen for the turbine are in an area of the T-s diagram where the slope is nearly vertical. Thus, the isentropic turbine process, starting at the saturated vapor line lies very close to the saturated vapor line for the entire process. This is verified by the calculation of the exit quality from the turbine, x4. To compute this quality we note that the ideal cycle has an isentropic turbine so that s 4 = s3 = sg(1.6 MPa) = 0.90784 kJ/kg∙K. At the condenser pressure of 0.7 MPa and an entropy of 0.90784 kJ/kg∙K, we find the quality as follows. x4 s 4 s f (0.7 MPa ) s fg (0.7 MPa ) 0.90784 kJ kg K 0.33230 kJ 0.58763 kJ kg K kg K xout = 0.979 . The value of 98.3% for quality confirms the turbine path in the diagram that is close to the saturated line for the entire process. Unit eleven homework solutions ME 370, L. S. Caretto, Fall 2010 Page 3 Temperature (K) Next, we do the usual set R-134a Rankine Cycle of calculations for the Rankine 400 cycle. In order to compute the 350 efficiency, we need the 300 enthalpy values at all the state 250 points. Following a conventional 200 Rankine cycle calculation, we find the Saturation Steam 150 properties at Generator state one as Pump Turbine those of a 100 saturated Condenser liquid at the condenser 50 pressure: h1 = hf(0.7 MPa) = 88.82 kJ/kg 0 and v1 = 0 0.5 1 1.5 2 0.0008331 Entropy (kJ/kg-K) m3/kg. The isentropic pump work, |wp| = v1(P2 – P1) where P2 is the same as the inlet pressure to the turbine, 1.6 MPa = 1600 kPa. Thus, 3 wP1 0.0008331 m 1600 kPa 700 kPa v1 P2 P1 kg 1 kJ kPa m 3 0.75 kJ kg We then find h2 = h1 + |wP1| = 86.78 kJ/kg + 0.75 kJ/kg = 89.57 kJ/kg. h3 = hg(1.6 MPa) = 277.863 kJ/kg. s3 = sg(1.6 MPa) = 0.90784 kJ/kg∙K. As noted above, state 4 is in the mixed region with P4 = 700 kPa and x4 = 0.979. We thus find the enthalpy from the quality as h4 = hf(P4 = 700 kPa) + x4 hfg(P4 = 700 kPa) = 88.82 kJ/kg + (0.979)(176.212 kJ/kg) or h4 = 261.41 kJ/kg. The heat intput to the steam generator, qh = h3 – h2 = 277.86 kJ/kg – 89.54 kJ/kg = 188.3 kJ/kg The condenser heat rejection, ql = |h1 – h4| = |88.62 kJ/kg – 261.41 kJ/kg| = 172.6 kJ/kg. The net work, w = qh - |qL| =188.3 kJ/kg| - 172.6 kJ/kg = 15.70 kJ/kg. The efficiency = w / qH = (15.70 kJ/k ) / (188.3 kJ/kg) or = 8.3% . The power output = 6 kg 15.71 kJ 1 kW W m w s kg kJ s W 94.2 kW Unit eleven homework solutions ME 370, L. S. Caretto, Fall 2010 Page 4 3. Consider a steam power plant that operates on a simple ideal Rankine cycle and has a net power output of 45 MW. Steam enters the turbine at 7 MPa and 500oC and is cooled in the condenser to a pressure of 10 kPa by running cooling water from a lake through the condenser at a rate of 2000 kg/s. Show the cycle on a T-s diagram with respect to the saturation lines, and determine (a) the thermal efficiency of the cycle, (b) the mass flow rate of the steam, and (c) the temperature rise of the cooling water. The diagram for this cycle is similar to the diagram for the cycle in problem 9-16 and is not shown here. In order to compute the efficiency, we need the enthalpy values at all the state points. Following a conventional Rankine cycle calculation, we find the properties at state one as those of a saturated liquid at the condenser pressure: h1 = hf(10 kPa) = 191.83 kJ/kg and v1 = 0.001010 m3/kg. The isentropic pump work, |wp| = v1(P2 – P1) where P2 is the same as the inlet pressure to the turbine, 7 MPa = 7,000 kPa. Thus, wP1 v1 P2 P1 0.001010 m 3 7000 kPa 10 kPa 1 kJ 3 7.060 kJ kg kPa m kg We then find h2 = h1 + |wP1| = 191.81 kJ/kg + 7.06 kJ/kg = 198.87 kJ/kg. h3 = h(7 MPa, 500oC) =3411.4 kJ/kg. s3 = s(7 MPa, 500oC) = 6.8000 kJ/kg∙K. h4 = h(P = Pcond = 10 kPa, s4 = s3). We see that this state is in the mixed region so we have to compute the quality to determine the enthalpy. x4 s 4 s f (10 kPa) s fg (10 kPa) 6.8000 kJ kg K 0.6492 kJ 7.4996 kJ kg K xout = 0.8202 . kg K With P4 = 10 kPa and x4 = 0.82027, we find the value of h4 = hf(P4 = 10 kPa) + x4 hfg(P4 = 10 kPa) = 191.81 + (0.8202)(2392.` kJ/kg) or h4 = 2153.7 kJ/kg. The heat input to the steam generator, qh = h3 – h2 = 3411.4 kJ/kg – 198.87 kJ/kg = 3212.5 kJ/kg The condenser heat rejection, ql = |h1 – h4| = |191.81 kJ/kg – 2153.7 kJ/kg| = 1961.8 kJ/kg. The net work, w = qh - |qL| = 3212.5 kJ/kg| - 1961.8 kJ/kg = 1250.6 kJ/kg. The efficiency = w / qH = (1250.6 kJ/kg) / (3212.5 kJ/kg) or = 38.9% . The mass flow, m 45 MW W w 1250.6 kJ kg 1000 kJ MW s m 35.98 kg s The heat rejection rate from the steam to the cooling water is the product of the mass flow rate m q 35.98 kg 1961.8 kJ 70591 kJ and the value of |qL|: Q L L s s s This heat is added to the cooling water. The cooling water flow is modeled as a steady flow with negligible kinetic and potential energies. There is no useful work. We model the enthalpy change of the cooling water by the equation h = cpT since we assume that the effect of pressure changes on the enthalpy of the relatively incompressible liquid water will be negligible. Applying the first law to the cooling water then gives the following relationship between the condenser heat rejection and the cooling water temperature rise, Tcw: Q cw Q L m cw hcw ,out hcw ,in m cw c p ,cw Tcw ,out Tcw ,in m cw c p ,cw Tcw Unit eleven homework solutions ME 370, L. S. Caretto, Fall 2010 Page 5 We can solve this equation for Tcw, and substitute in the given values including the heat capacity of liquid water to give cp,cw = 4.18 kJ/kg∙K (Table A-3(a) on page 914 of the text for liquid water at 25oC) to obtain the final answer for the temperature rise. Tcw Q L m cw c p ,cw 70591 kJ s 2000 kg 4.18 kJ s kg K Tcw = 8.44oC . 4. A steam power plant operates on an ideal regenerative Rankine cycle. Steam enters the turbine at 6 MPa and 450oC and is condensed in the condenser at 20 kPa. Steam is extracted from the turbine at 0.4 MPa to heat the feedwater in an open feedwater heater. Water leaves the feedwater heater as a saturated liquid. Show the cycle on a T-s diagram and determine (a) the net work per kilogram of steam flowing through the boiler and (b) the thermal efficiency of the cycle. High Pressure Turbine (T1) The diagram of the components in the cycle is shown on the left. In terms of the numbered points on this diagram, the input data for the problem give P5 = 6 MPa, T5 = 450oC, Pcond = 20 kPa, and PFWH = 0.4 MPa. Low Pressure Turbine (T2) 5 7 Steam Generator 8 6 Feedwater Heater 3 4 2 Pump (P2) Condenser 1 Pump (P1) For the ideal cycle in which there are no line losses in pressure or temperature and no pressure drops in heat transfer devices, we have P4 = P5,= 6 MPa, P2 = P3 = P6 = P7 = PFWH = 0.4 MPa, and P1 = P8 = Pcond = 20 kPa. The ideal cycle has isentropic work devices so s8 = s7 = s6 = s5; s2 = s1 and s4 = s3. Finally points 1 and 3 are saturated liquid. As usual, we assume that the individual components are steady-flow devices with negligible kinetic and potential energies. There is no useful work in the steam generator, feedwater heater, or condenser. The turbine and pumps are reversible and adiabatic meaning that there is no heat transfer or entropy change. Thus the first law for each device only one inlet and one outlet is q = w + hout – hin. We begin by determining the enthalpy at each point in the cycle. The properties at state one as those of a saturated liquid at the condenser pressure: h 1 = hf(20 kPa) = 251.42 kJ/kg and v1 = 0.001017 m3/kg. The pumps are isentropic and we calculate the work of the first pump as follows: |wp1| = v1(P2 – P1). Thus, wP1 v1 P2 P1 0.001017 m 3 400 kPa 20 kPa 1 kJ 3 0.39 kJ kg kg kPa m We then find h2 = h1 + |wP1| = 251.42 kJ/kg + 0.39 kJ/kg = 251.80 kJ/kg. The properties at state three are also those of a saturated liquid. Here the pressure is the feedwater heater pressure so that h3 = hf(400 kPa) = 604.66 kJ/kg and v3 = 0.001084 m3/kg. We use the vP calculation for isentropic pump work for the second pump. Unit eleven homework solutions wP2 v3 P4 P3 ME 370, L. S. Caretto, Fall 2010 Page 6 0.001084 m 3 6000 kPa 400 kPa 1 kJ 3 6.07 kJ kg kg kPa m We then find h4 = h3 + |wP2| = 604.66 kJ/kg + 6.07 kJ/kg = 610.73 kJ/kg. h5 = h(6 MPa, 450oC) = 3302.9 kJ/kg. s5 = s(6 MPa, 450oC) =6.7219 kJ/kg∙K. h6 = h(P = PFWH = 400 kPa, s6 = s5). We see that this state is in the mixed region so we have to compute the quality to determine the enthalpy. x6 s 6 s f (400 kPa) s fg (400 kPa) 6.7219 kJ kg K 1.7765 kJ 5.1191 kJ kg K 0.9661 kg K h6 = hf(P6 = 400 kPa) + x6 hfg(P6 = 400 kPa) = 604.66 kJ/kg + (0.9661)(2133.4 kJ/kg) or h6 = 2665.7 kJ/kg. State 7 is the same as state 6 so we have h7 = 2665.7 kJ/kg. h8 = h(P = Pcond = 20 kPa, s8 = s5). We see that this state is in the mixed region so we have to compute the quality to determine the enthalpy. x8 s8 s f (20 kPa) s fg (20 kPa) 6.7219 kJ kg K 0.8320 kJ 7.0752 kJ kg K 0.8325 kg K h8 = hf(P8 = 20 kPa) + x8 hfg(P8 = 20 kPa) = 251.42 kJ/kg + (0.8325)(2357.5 kJ/kg) or h8 = 2213.97 kJ/kg. In this cycle there are three distinct mass flow rates at different points in the cycle. These are 6 a represents the mass flow into the feedwater heater.) shown in the equations below. (Here, m m 3 m 4 m 5 m a m 7 m 8 m 1 m 2 m b 6a m 3 m 2 m a m b m Taking a mass and energy balance around the feedwater heater gives the following relation for the mass flow ratio. We can substitute the enthalpy values found above to compute this ratio. 604.66 kJ 2665.67 kJ m m 2 h3 h6 kg kg 0.8538 b 251.80 kJ 2665.6 kJ m 3 h2 h6 m a kg kg We can compute the heat input rate for the steam generator, using m 4 m 5 m a as the mass flow rate in the steam generator. Q SG m a h5 h4 The power output from the two turbine stages is given by the following equation, which accounts for the differences in mass flow rate in the two stages. WT m a h5 h6 m b h7 h8 Unit eleven homework solutions ME 370, L. S. Caretto, Fall 2010 Page 7 Finally, the total power input to the pumps is computed by accounting for the differences in mass flow rates. W P m a wP2 m b wP1 We now have the necessary information to compute the cycle efficiency. WT W P W net m a h5 h6 m b h7 h8 m a wP2 m b wP1 m a h5 h4 Q SG Q SG We can divide by the mass flow rate, a to get the following equation for the efficiency in terms m of the mass flow rate ratio that we found from our analysis of the feedwater heater. W net h5 h6 mb h7 h8 wP2 mb wP1 m m a m a a h5 h4 QSG m a In this form, the numerator of the efficiency equation is the net work per unit mass flowing through the steam generator. W net m m h5 h6 b h7 h8 wP2 b wP1 m a m a m a Substituting the values found for the enthalpies in the cycle and the mass flow rate ratio gives the net work per unit mass flowing through the steam generator as follows: W net 3302.9 kJ 2665.7 kJ 2665.7 kJ 2214.0 kJ (0.8538) m a kg kg kg kg 6.07 kJ 0.39 kJ (0.8538) kg kg W net 1016.5 kJ m a kg From the equations for the efficiency and the net work, we see that we can use the computed value of work to simplify the efficiency calculation. W net m m W net 1016.5 kJ h5 h6 b h7 h8 wP2 b wP1 m m a m a m a kg a h5 h4 h5 h4 3302.9 kJ 610.73 kJ Q SG kg kg m a = 37.8% Unit eleven homework solutions ME 370, L. S. Caretto, Fall 2010 Page 8 5. Repeat problem 4 with the open feedwater heater replaced by a closed feedwater heater. Assume that the feedwater leaves the heater at the condensation temperature of the extracted steam and that the extracted steam leaves the heater as a saturated liquid and is pumped to the line carrying the feedwater. The diagram of the components in the cycle is shown on the left. In the closed feedwater heater, the feed water flows from point w to point 4, without mixing with the extracted steam. (The steam enters at point 8, transfers heat to the feed water without mixing, and leaves at point 3. 7 Steam Generator High Pressure Turbine (T1) Low Pressure Turbine (T2) 9 10 8 6 4 Feedwater Heater Mixing Chamber 3 2 5 Condenser 1 Pump (P1) Pump (P2) In terms of the numbered points on this diagram, the input data for the problem give P7 = 6 MPa, T7 = 450oC, Pcond = 20 kPa, and P8 = 0.4 MPa. We are also told that point 3 is a saturated liquid and T4 has the same temperature as this saturated liquid For the ideal cycle in which there are no line losses in pressure or temperature and no pressure drops in heat transfer devices, we have P2 = P4 = P5 = P6 = P7 = 6 MPa, P3 = P8 = 0.4 MPa, and P1 = P10 = Pcond = 20 kPa. The ideal cycle has isentropic work devices so s10 = s9 = s8 = s7; s2 = s1 and s5 = s3. Finally points 1 and 3 are saturated liquid. In this cycle there are three distinct mass flow rates at different points in the cycle. These are 8 a represents the mass flow into the feedwater heater.) shown in the equations below. (Here, m m 6 m 7 m a m 9 m 10 m 1 m 2 m 4 m b m 8a m 3 m 5 m c Taking a mass balance around the mixing chamber gives the following relation among the three mass flow rates. m 6 m 4 m 5 m a m b m c As usual, we assume that the individual components are steady-flow devices with negligible kinetic and potential energies. There is no useful work in the steam generator, feedwater heater, or condenser. The turbine and pumps are reversible and adiabatic meaning that there is no heat transfer or entropy change. Thus the first law for each device with only one inlet and one outlet is q = w + hout – hin. We begin by determining the enthalpy at each point in the cycle. The properties at state one as those of a saturated liquid at the condenser pressure: h 1 = hf(20 kPa) = 251.42 kJ/kg and v1 = 0.001017 m3/kg. The pumps are isentropic and we calculate the work of the first pump as follows: |wp1| = v1(P2 – P1). Thus, wP1 v1 P2 P1 0.001014 m 3 6000 kPa 20 kPa 1 kJ 3 6.08 kJ kg kPa m kg We then find h2 = h1 + |wP1| = 251.42 kJ/kg + 6.08 kJ/kg = 257.50 kJ/kg. Unit eleven homework solutions ME 370, L. S. Caretto, Fall 2010 Page 9 The properties at state three are also those of a saturated liquid. Here the pressure is the feedwater heater pressure so that h3 = hf(400 kPa) = 604.66 kJ/kg and v3 = 0.001084 m3/kg. We use the vP calculation for isentropic pump work for the second pump. wP2 v3 P5 P3 0.001084 m 3 6000 kPa 400 kPa 1 kJ 3 6.07 kJ kg kPa m kg We then find h5 = h3 + |wP2| = 604.66 kJ/kg + 6.07 kJ/kg = 610.73 kJ/kg. According to the problem information T4 has the same temperature as the saturated liquid at point three. From the saturation tables we find this temperature as 143.61oC. This is a compressed liquid and we can use the following data in the compressed liquid. We can use a double interpolation in the compressed liquid tables to find the enthalpy at this point. First we use two interpolations to find the enthalpy at the desired temperature of 143/61 oC at the two pressures bounding the given pressure of 6 MPa in the tables. h(143.61o C ,5 MPa) 592.18 kJ kg h(143.61o C ,10 MPa) 672.55 kJ 592.18 kJ kg kg 160 C 140 C 681.01 kJ 595.45kJ kg kg o 595.45 kJ kg o 160 C 140 C o o 143.61 C 140 C 606.kg68 kJ o o 143.61 C 140 C 610.kg89 kJ o o We can now use these two values to find the desired enthalpy at 6 MPa. 610.89 kJ 606.68 kJ 606.68 kJ kg kg 6 MPa 5 MPa 607.53 kJ h4 kg 10 MPa 5 MPa kg The mixing chamber has no heat or work, but is has three different mass flow rates. Thus the first law and mass conservation equations for this device can be written as shown below and manipulated to get an equation for h6 in terms of mass flow rate ratios. m 6 m 4 m 5 m 6 h6 m 4 h4 m 5h5 m a m b m c h6 m b m c 1 m a m a m m m m 4 h4 5 h5 b h4 1 b h5 m 6 m 6 m a m a Thus, we can compute h6 if we know the mass flow rate ratio in the above equation. We can find this mass flow rate ratio from an analysis of the closed feedwater heater. Application of the first law for no heat and work (and recognizing that the two streams in this device do not mix) gives the following result. m 2 h2 m 8a h8 m 3 h3 m 4 h4 m b h2 m c h8 m c h3 m b h4 m b h3 h8 m c h2 h4 We have already seen how to compute h1, h3, and h4, and we will determine h8 below. Thus we will be able to compute the mass flow rate ratio compute the ratio m b m b shown above from enthalpy values. To m c required to compute h6, we have to make the following computations. m a Unit eleven homework solutions ME 370, L. S. Caretto, Fall 2010 Page 10 m b m b m b h3 h8 m b m c m c m c h2 h4 h3 h8 m a m a m b m c m b 1 1 m c m c m c h2 h4 We continue to find enthalpy values, using the conventional methods for the isentropic turbine work. h7 = h(6 MPa, 450oC) = 3302.9 kJ/kg. s7 = s(6 MPa, 450oC) =6.7219 kJ/kg∙K. h8 = h(P = P8 = 400 kPa, s8 = s7). We see that this state is in the mixed region so we have to compute the quality to determine the enthalpy. x8 s8 s f (400 kPa) s fg (400 kPa) 6.7219 kJ kg K 1.7765 kJ 5.1191 kJ kg K 0.9661 kg K h8 = hf(P8 = 400 kPa) + x8 hfg(P8 = 400 kPa) = 604.66 kJ/kg + (0.9661)(2133.4 kJ/kg) or h8 = 2665.67 kJ/kg. State 9 is the same as state 9 so we have h9 = 2665.67 kJ/kg. h10 = h(P = Pcond = 20 kPa, s10 = s7). We see that this state is in the mixed region so we have to compute the quality to determine the enthalpy. x10 s10 s f (20 kPa) s fg (20 kPa) 6.7219 kJ kg K 0.8320 kJ 7.0752 kJ kg K 0.8325 kg K h10 = hf(P10 = 20 kPa) + x10 hfg(P10 = 20 kPa) = 251.42 kJ/kg + (0.8325)(2357.5 kJ/kg) or h10 = 2213.97 kJ/kg. We now have all the enthalpy values required to compute the mass flow rate ratios 604.66 kJ 2665.67 kJ m b h3 h8 kg kg 5.888 257.50 607.53 kJ m c h2 h4 kg kg mb m b m b m c m c m a 0.8548 5.888 0.8548 0.14158 m a m b m c 5.888 1 m a m b 5.888 m c m c With the value just found for h6 m m b h4 1 b m a m a m b , we can compute h6: m a 607.52 kJ 610.73 kJ 607.99 kJ h5 (0.8548) 1 0.8548 kg kg kg Unit eleven homework solutions ME 370, L. S. Caretto, Fall 2010 We can compute the heat input rate for the steam generator, using Page 11 m 6 m 7 m a as the mass flow rate in the steam generator. Q SG m a h7 h6 The power output from the two turbine stages is given by the following equation, which accounts for the differences in mass flow rate in the two stages. WT m a h7 h8 m b h9 h10 Finally, the total power input to the pumps is computed by accounting for the differences in mass flow rates. W P m c wP2 m b wP1 We now have the necessary information to compute the cycle efficiency. WT W P W net m a h7 h8 m b h9 h10 m c wP2 m b wP1 m a h7 h6 Q SG Q SG We can divide by the mass flow rate, a to get the following equation for the efficiency in terms m of the mass flow rate ratio that we found from our analysis of the feedwater heater. W net h7 h8 mb h9 h10 wP2 mb wP1 m m a m a a h7 h6 QSG m a In this form, the numerator of the efficiency equation is the net work per unit mass flowing through the steam generator. W net m m m h7 h8 b h9 h10 c wP2 b wP1 m a m a m a m a Substituting the values found for the enthalpies in the cycle and the mass flow rate ratio gives the net work per unit mass flowing through the steam generator as follows: W net 3302.9 kJ 2665.7 kJ 2665.7 kJ 2214.0 kJ (0.8548) m a kg kg kg kg 6.07 kJ 6.08 kJ 0.14518 (0.8548) kg kg W net 1017.3 kJ m a kg Unit eleven homework solutions ME 370, L. S. Caretto, Fall 2010 Page 12 From the equations for the efficiency and the net work, we see that we can use the computed value of work to simplify the efficiency calculation. W net W net 1017.3 kJ h7 h8 mb h9 h10 mc wP2 mb wP1 m m a m a m a m a kg a h7 h6 h5 h4 3302.9 kJ 607.99 kJ QSG kg kg m a = 37.7% 6 A steam power plant operates on an ideal reheat-regenerative Rankine cycle and has a net power output of 80 MW. Steam enters the high-pressure turbine at 10 MPa and 550oC and leaves at 0.8 MPa. Some of the steam is extracted at this pressure to heat the feedwater in an open feedwater heater. The rest of the steam is reheated to 500 oC and is expanded in the low pressure turbine to the condenser pressure of 10 kPa. Show the cycle on a T-s diagram and determine (a) the mass flow rate of steam flowing through the boiler and (b) the thermal efficiency of the cycle. High Pressure Turbine (T1) Low Pressure Turbine (T2) 5 6 7 Steam Generator 8 Feedwater Heater 3 4 The diagram of the components in the cycle is shown on the left. In terms of the numbered points on this diagram, the input data for the problem give P5 = 10 MPa, T5 = 550oC, Pcond = 10 kPa, and PFWH = 0.8 MPa, and T7 = 500oC. 2 Pump (P2) Condenser 1 Pump (P1) For the ideal cycle in which there are no line losses in pressure or temperature and no pressure drops in heat transfer devices, we have P4 = P5,= 10 MPa, P2 = P3 = P6 = P7 = PFWH = 0.8 MPa, and P1 = P8 = Pcond = 20 kPa. The ideal cycle has isentropic work devices so s8 = s7, s6 = s5; s2 = s1 and s4 = s3. Finally points 1 and 3 are saturated liquid. As usual, we assume that the individual components are steady-flow devices with negligible kinetic and potential energies. There is no useful work in the steam generator, feedwater heater, or condenser. The turbine and pumps are reversible and adiabatic meaning that there is no heat transfer or entropy change. Thus the first law for each device only one inlet and one outlet is q = w + hout – hin. We begin by determining the enthalpy at each point in the cycle. The properties at state one as those of a saturated liquid at the condenser pressure: h 1 = hf(10 kPa) = 191.81 kJ/kg and v1 = 0.001010 m3/kg. The pumps are isentropic and we calculate the work of the first pump as follows: |wp1| = v1(P2 – P1). Thus, wP1 v1 P2 P1 0.001010 m 3 800 kPa 10 kPa 1 kJ 3 0.80 kJ kg kPa m kg We then find h2 = h1 + |wP1| = 191.81 kJ/kg + 0.80 kJ/kg = 192.61 kJ/kg. Unit eleven homework solutions ME 370, L. S. Caretto, Fall 2010 Page 13 The properties at state three are also those of a saturated liquid. Here the pressure is the feedwater heater pressure so that h3 = hf(800 kPa) = 720.87 kJ/kg and v3 = 0.001115 m3/kg. We use the vP calculation for isentropic pump work for the second pump. wP2 v3 P4 P3 0.001115 m 3 10000 kPa 800 kPa 1 kJ 3 10.26 kJ kg kPa m kg We then find h4 = h3 + |wP2| = 720.87 kJ/kg + 10.26 kJ/kg = 731.13 kJ/kg. h5 = h(10 MPa, 550oC) = 3502.0 kJ/kg. s5 = s(1 MPa, 550oC) =6.7585 kJ/kg∙K. h6 = h(P = PFWH = 800 kPa, s6 = s5). This state is in the gas region so we have to find h6 by interpolation between the first two rows at 800 kPa. This gives h6 = 2812,8 kJ/kg. h7 = h(0.8 MPa, 500oC) = 3481.3 kJ/kg. s7 = s(0.8 MPa, 500oC) =7.8692 kJ/kg∙K. h8 = h(P = Pcond = 10 kPa, s8 = s7). We see that this state is in the mixed region so we have to compute the quality to determine the enthalpy. x8 s8 s f (10 kPa) s fg (10 kPa) 7.8692 kJ kg K 0.6492 kJ 7.4996 kJ kg K 0.9627 kg K h8 = hf(P8 = 10 kPa) + x8 hfg(P8 = 10 kPa) = 191.81 kJ/kg + (0.9627)(2392.1 kJ/kg) or h8 = 2494.7 kJ/kg. In this cycle there are three distinct mass flow rates at different points in the cycle. These are 6 a represents the mass flow into the feedwater heater.) shown in the equations below. (Here, m m 3 m 4 m 5 m a m 7 m 8 m 1 m 2 m b 6a m 3 m 2 m a m b m Taking a mass and energy balance around the feedwater heater gives the following relation for the mass flow ratio. We can substitute the enthalpy values found above to compute this ratio. 720.87 kJ 2812,8 kJ m m 2 h3 h6 kg kg 0.7984 b m 3 h2 h6 192.61 kJ 2812.8 kJ m a kg kg We can compute the heat input rate for the steam generator, using m 4 m 5 m a as the mass flow rate for the initial the steam generator flow and mb for the reheat flow. Q SG m a h5 h4 m b h7 h6 The power output from the two turbine stages is given by the following equation, which accounts for the differences in mass flow rate in the two stages. WT m a h5 h6 m b h7 h8 Finally, the total power input to the pumps is computed by accounting for the differences in mass flow rates. Unit eleven homework solutions ME 370, L. S. Caretto, Fall 2010 Page 14 W P m a wP2 m b wP1 We now have the necessary information to compute the cycle efficiency. WT W P W net m a h5 h6 m b h7 h8 m a wP2 m b wP1 m a h5 h4 m b h7 h6 Q SG Q SG We can divide by the mass flow rate, a to get the following equation for the efficiency in terms m of the mass flow rate ratio that we found from our analysis of the feedwater heater. W net h5 h6 mb h7 h8 wP2 mb wP1 m m a m a a QSG h5 h4 mb h7 h6 m a m a In this form, the numerator of the efficiency equation is the net work per unit mass flowing through the steam generator. W net m m h5 h6 b h7 h8 wP2 b wP1 m a m a m a Substituting the values found for the enthalpies in the cycle and the mass flow rate ratio gives the net work per unit mass flowing through the steam generator as follows: W net 3502.0 kJ 2812.8 kJ 3481.3 kJ 2494.7 kJ (0.7984) m a kg kg kg kg 10.26 kJ 0.80 kJ 1465.9 kJ (0.7984) kg kg kg From this specific work, we can find the mass flow rate required for a power output of 80 MW. 1000 kJ 80 MW Wnet MW s m a 1465.9 kJ W net m a kg m a 54.6 kg s From the equations for the efficiency and the net work, we see that we can use the computed value of work to simplify the efficiency calculation. W net W net h5 h6 mb h7 h8 wP2 mb wP1 m m a m a m a a QSG h5 h4 mb h7 h8 h5 h4 mb h7 h8 m a m a m a Unit eleven homework solutions ME 370, L. S. Caretto, Fall 2010 1465.9 kJ kg 3481.3 kJ 2812.8 kJ 3502.0 kJ 731.13 kJ (0.7984) kg kg kg kg Page 15 = 44.4% An alternative approach for finding the efficiency is to determine the heat loss in the condenser. Q cond m b h1 h8 . Since this is the rejected heat, we can use the following approach for computing the efficiency. m b h1 h8 QSG Qcond Wnet m b h1 h8 m a 1 1 m m a h5 h4 m b h7 h6 Q SG Q SG h5 h4 b h7 h6 m a Applying the results previously found to this equation gives... 2494.7 kJ 191.81 kJ (0.7984) kg kg 1 44.4% 3481.3 kJ 2812.8 kJ 3502.0 kJ 731.13 kJ (0.7984) kg kg kg kg