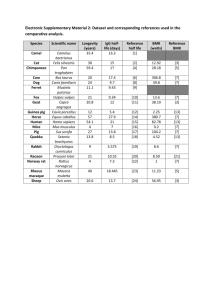
Read full article
advertisement

TITLE: DETECTION OF ANTI-RABIES NEUTRALIZING ANTIBODIES IN
HUMANS AND CATTLE BY A COMBINED TISSUE CULTURE AND ENZYME
LINKED IMMUNOASSAY*
AUTHORS: D. Katz*, E. Freeman*1, M. Davidson**, M. Abramson** and P. Fuchs*
*. Department of Infectious Diseases, Israel Institute for Biological Research, Ness-Ziona, Israel.
**. Veterinary Services, Ministry of Agriculture, Beit-Dagan, Israel
Summary
We have developed a tissue culture neutralization assay based on enzyme
immunoassay - NTCEIA, which enables rapid determination of the neutralizing antirabies antibody titer of a large number of sera at the same time. The test is performed
in plastic microtiter plates and comprises three steps: Neutralization performed in the
wells of these plates; The infection step where cells are seeded into the wells to be
infected by the non-neutralized virus fraction and the identification step where cells
are fixed and the replicating virus is identified by enzyme immunoassay. By omitting
the neutralization step, steps 2 and 3 of this test can be used for viral antigen titration.
The test is rapid, easy to perform, highly reproducible and shows a good correlation to
the standard mouse neutralization test. These features make NTCEIA suitable for
large-scale serosurveys. The test can be easily adapted to any animal species and we
demonstrate here the advantages of the test in a small-scale study of the efficacy of
anti-rabies vaccination in calves and cows.
Introduction
Rabies, which is endemic in Israel, is found every year among household, farm and
wild animals. Usually the disease is sylvatic where the number of affected wild
animals is highest. An alarming fact is that the incidence of the disease is rising and in
1997 a record number of 83 rabid animals were reported by the Israeli Veterinary
Services. These included 8 dogs, 57 foxes, 2 jackals and 5 cases in cattle. The
reported cases are not limited to certain focal areas but were distributed all over the
country. It can be therefore assumed that the reported numbers, based on the
incidental collection of rabid animals, are just the tip of an iceberg and represent a
much higher incidence of the disease. In 1996-7, three fatal human cases were
diagnosed following three decades without any human case.
Farm animals and mainly grazing cattle and sheep in Israel are exposed to rabid
animals including foxes, stray dogs and jackals. Rabies control in Israel is based on
mandatory vaccination of dogs and voluntary immunization of farm animals, mainly
cows. The Rabies Order imposes six months quarantine on a dairy farm where a case
of rabies was confirmed and cows were not immunized. Three months quarantine is
imposed on an immunized farm. This causes heavy economic losses to the affected
farm and stresses the need for a reliable method to assess the immune status of
immunized herds.
The rise in rabies incidence has prompted the Israeli Veterinary Services to consider
the strategy, which was successful in Europe, to eradicate or suppress rabies by oral
vaccination combined with controlled destruction of wild animals (1,2). Such a
campaign is dependent on an efficient method to monitor the immune status of target
animals for the seroepidemiological surveillance.
We have conducted a survey to assess the efficiency of vaccination of Israeli cattle.
For this survey we previously developed an ELISA for rapid screening of anti-rabies
antibody titers in vaccinated cattle (3). However, this test was not as sensitive as the
classic mouse neutralization test (MNT) for assessing the immune status of vaccinated
animals. High ELISA titers corresponded to high neutralization titers but about 40%
of cattle negative by ELISA titer were positive by MNT.
The MNT, which more accurately reflects the immune status of the vaccinated
animals, is laborious and costly and is not suitable for field surveys. An alternative is
the rapid fluorescent focus inhibition technique (RFFIT) in which anti-rabies
antibodies are detected by inhibition of microscopic foci of virus- infected cells
stained by immunofluorescence. This method demands hours of tedious microscopic
observation and is also not suitable for large scale serosurveys.
An easier, more accurate and faster method is one which combines the advantages of
a “solid phase” immunoassay (RIA or ELISA) with the sensitivity of an assay which
monitors the infectivity of the virus in tissue culture. Such an assay was developed in
our laboratory for the detection of arbovirus antibodies (4) and by others for several
human viruses including one developed by Mannen et al for anti-rabies antibodies in
humans (5-8).
Here we report the adaptation of this assay for the detection of anti-rabies antibodies
in cattle sera. Neutralization tissue culture enzyme immuno assay (NTCEIA)
combines the infection of cells, grown in microplates, by the fraction of nonneutralized virus and its identification by ELISA (TCEIA). An ELISA reader
automatically monitors the results permitting objective quantitation of titers.
Materials and Methods
Cells:
BHK-21 cells and NSKSH cells (human neuroblstoma cell line, ATCC) were grown
in 96-well plastic microtiter plates in DMEM medium supplemented with 10% fetal
calf serum (MM-10FCS).
Virus:
Challenge virus standard (CVS, ATCC) strain of rabies virus was prepared as 5%
mouse brain suspension in Phosphate Buffered Saline (PBS). Virus titer was 4 X 106
MICLD50/ml (mouse intracerebral lethal dose).
Vaccines:
Calves and cows were vaccinated with “Rabicine” inactivated veterinary vaccine
(Merieux).
Sera:
International human Rabies immunoglobulin preparation (IHRIGP) was obtained
from the laboratories of the Ministry of Health, Jerusalem, as supplied by Staten
Serum Institute, Copenhagen, Denmark.
Standard cattle serum (SCAS) was obtained by pooling 7 sera taken from cows
immunized twice, at a 14-month interval, with Rabicine vaccine. Blood was drawn 2
weeks following the second vaccination and the sera were selected for their high titers
by ELISA and MNT.
Hyperimmune sera were obtained by immunizing rabbits with Merieux inactivated
human rabies vaccine. The vaccine was diluted 1:6 in saline and mixed with equal
volume of complete Freund’s adjuvant (CFA). 3 ml of such vaccine were injected
intramusculary and intradermally into young (1.5 kg) rabbits at several sites. The
rabbits received booster injections 3 weeks later and were bled several times from day
8 through 9 weeks thereafter.
Immunoezymatic assay reagents:
Alkaline phosphatase-coupled goat anti-rabbit IgG (GAR-AP) and Protein A-alkaline
phosphatase conjugate (PA-AP) (Sigma). The substrate was dinitro-phenyl phosphate
(Sigma).
Mouse Neutralization Test (MNT)
MNT was performed as published by Atanasiu (9) incorporating the International
human Rabies immunoglobulin preparation (Staten Serum Institute, Copenhagen,
Denmark).
Tissue culture neutralization assay (NTCEIA)
In its final form 12 antisera can be tested in one 96-well microtiter plate. Each serum
is tested in two duplicate dilutions and on each plate, six duplicate dilutions of
standard serum, which serve as a calibration curve, are included. Ten wells are
infected with the selected virus dilution to establish the optimal growth needed for
calculating of the neutralization antibody titer. Ten additional wells with non-infected
cells serve as background control.
The test is performed by mixing in the microtiter wells, 50 µl of virus (X 2
concentration) with 50 µl of each dilution of the tested serum in MM-10FCS.
Following 1 hr incubation at 370C each well is seeded with 40,000 cells. Acetone
(80%) fixation is
performed 48 hrs later (see Results). The wells are then incubated for 1 hr with
alkaline phosphatase conjugate followed by 30 min incubation with the substrate and
the OD is read at 405 nm.
Results
Optimization of the test for virus titration (TCEIA)
The neutralization Tissue Culture Enzyme Immunoassay (NTCEIA) has three steps.
The first is the neutralization of a constant dose of virus by incubation with the test or
standard antiserum. The second is inoculation of cells in microplates with the virusantiserum mixture and the third is to establish infection by ELISA, following fixation
of the cells.
Steps 2 and 3 comprise a method for infectious virus titration - TCEIA. This test had
to be developed first so that the concentration of the virus can be determined for use
in NTCEIA.
The optimal time for fixation of the cells was 48 hours post-infection and the best
fixative was 80% acetone. We compared two cell lines, BHK-21 and SKNSH human
neuroblstoma cell line, for their performance in TCEIA. Figure 1 summarizes an
experiment where they were infected with the same virus inoculum (1:180 dilution in
MM-10FCS) and following 48h incubation at 370C the cells were fixed and incubated
for 1h with rabbit anti-rabies serum (1:100 dilution in PBS + 0.05% Tween + 0.5%
BSA). In this experiment two brands of microtiter plates (Costar and Nunc) and two
anti-rabbit IgG conjugates – GAR-AP and PA-AP were compared (see materials and
methods). As can be seen SKNSH cells performed in all cases significantly better than
BHK. Both GAR-AP and PA-AP conjugates were suitable, although with human
serum the PA-AP was slightly more efficient. There was no difference between the
two brands of plates.
To establish the optimal concentration of virus for NTCEIA a calibration titration was
performed. To virus dilutions in quadruplicate wells, SKNSH cells were added.
Following 48h incubation at 370C and acetone fixation, two of the four wells were
incubated with rabbit anti-rabies serum and the other two wells served as controls and
were incubated with normal rabbit serum (NRS, 1:100 dilution). The conjugate was
PA-AP (1:1500 dilution in PBS + 0.05% Tween + 0.5% BSA). The results are shown
in Figure 2. From the exponential curve obtained the optimal virus concentration for
use in the neutralization test was established at 1:180 dilution. This gave a significant
OD signal suitable for the interpreting the neutralization assay.
If we consider as positive the dilution of the virus which gave an OD reading double
that of the NRS, the titer of the virus is 4X104 MICLD50/50 µl or 8X105
MICLD50/ml. If this is compared with the titer of the mouse infectious seed virus
(4X106 MICLD50/ml) the conclusion is that one TCEIA unit is about 5 MICLD50/ml.
NTCEIA for International Standard Human Serum (IHRIGP) and Standard
Cattle Serum (CSAS)
The neutralization test was first performed with the human IHRIGP with a known
concentration (expressed as International Units –IU) of neutralizing anti-rabies
antibodies.
IHRIGP was diluted in two fold steps (1:50-1:800 in MM-10FCS) in microtiter plates
and incubated in duplicate with 1:90 dilution of virus (final dilution of virus – 1:180).
Following incubation with the rabbit anti-rabies serum (1:100 dilution) cells were
incubated with GAR-AP conjugate (1:1000 dilution in PBS + 0.05% Tween + 0.5%
BSA). Two controls were included: C1- background control for the rabbit antiserum
(non-infected cells incubated with the rabbit anti-rabies); C-2 – virus control (cells
infected with non-neutralized virus diluted 1:180).
Percent neutralization (%NT) by an antiserum dilution (Diln) was calculated as
follows:
%NT = 100-{[(Diln-C1) / (C2-C1)] X 100}
Where Diln, C1 and C2 represent the average OD in the corresponding wells.
The results of this experiment are depicted in Figure 3 in which the %NT is plotted as
a function of serum dilution. The titer of the antiserum is determined as the reciprocal
of the dilution at the 50% neutralization point. The titer of the IHRIGP in this test is
278. As the starting concentration of the IHRIGP in the test was 10 IU/ml, a titer of
278 is equal therefore to 10 IU/ml. This ratio enables us to express the neutralization
titer in International Units equivalent.
A similar titration of SCAS was performed (Figure 3).
In Figure 4 the %NT obtained with the cattle standard serum was plotted against the
logarithm of the reciprocal of the serum dilution. Using regression analysis, the
expected percent neutralization curve (from the values obtained in the experiment)
was also plotted. This curve is very close to the experimental one thus demonstrating
the linear correlation between log dilution and %NT (correlation coefficient, r = 0.91).
The 50% neutralization point in the experiment depicted in Figure 4 is 3.29 log
reciprocal of dilution and the titer of the standard cattle serum is therefore 1947 cattle
neutralization units (CNTU, the antilog of 3.29).
The titer of a given antiserum can be calculated provided that its neutralization titer is
on the linear part of the curve. For each serum sample tested we therefore include two
dilutions so that at least one of them will fall within the linear part of the curve.
Reproducibility of NTCEIA
The reproducibility of the test was calculated from 16 separate titrations of IHRIGP
and 17 titrations of the standard cattle serum. The results summarized in Table 1 show
good test reproducibility for both sera. The starting concentration of IHRIGP was 10
IU/ml, and the titer of 278 (Table 1) obtained for this serum means that 1 IU equals 28
neutralization units in the NTCEIA. The titer of the standard cattle serum equals 79
IU (2187 X 10/278).
Table 1 : Reproducibility of NTCEIA for International Human Rabies
Immunoglobulin Preparation (IHRIGP) and cattle standard serum.
Serum
Number of
repetitions
(n)
Mean Titer
+ 1 standard
deviation
coef.
variation
(%)
IHRIGP
16
278 (NTU)
67 (NTU)
24
CSAS
17
2178 (CNTU)
376 (CNTU)
Comparison between NTCEIA and mouse neutralization test (MNT)
Sera from 45 immunized cows were tested in both NTCEIA and MNT. The results of
both tests, expressed as International Units for the MNT and Cattle Neutralizing Units
(CNTU) for NTCEIA, are summarized in Table 2.
A good correlation between the two tests was obtained. Four sera, however, gave a
higher factor in MNT than in NTCEIA and three gave a lower factor.
Table 2 : comparison of anti rabies antibody titer obtained by MNT and NTCEIA in
45 bovine sera
Serum
NTCEA Serum
Titer
(CNTU)
#
MNT
Titer
(IU)
NTCEA Serum
Titer
(CNTU)
#
#
MNT
Titer
(IU)
MNT
Titer
(IU)
NTCEA
Titer
(CNTU)
1
50.0
97.6
16
61.3
39.6
31
1.6
0.8
2
8.5
10.4
17
>50.0
162.9
32
8.1
3.2
3
0.2
0.0
18
50.0
43.2
33
40.0
9.9
4
50.0
25.7
19
0.0
0.0
34
0.0
0.0
5
3.4
3.1
20
33.0
36.9
35
0.0
0.0
6
0.3
0.0
21
9.8
14.1
36
12.0
38.9
7
10.0
13.9
22
1.6
3.7
37
0.0
0.0
8
6.7
8.6
23
3.6
1.8
38
0.4
0.0
9
2.8
2.4
24
1.3
1.9
39
2.4
29.9
10
2.5
4.0
25
1.4
1.8
40
22.3
15.6
11
>50.0
349.0
26
70.0
19.5
41
18.2
7.2
12
>50.0
288.0
27
40.0
10.4
42
5.5
5.1
13
10
76.0
28
0.0
0.0
43
0.1
0.0
14
>50.0
107.0
29
5.0
9.1
44
0.0
0.0
15
13.6
19.4
30
1.6
10.0
45
0.0
0.0
The immune response of cattle vaccination in Israel measured by NTCEIA
Twenty eight calves from farm M were vaccinated with Rabicine vaccine. The calves
were divided into three groups: Nine calves were immunized subcutanously, 9
intramuscularly and 10 were immunized subcutanously with Rabicine + FMDV (Foot
and Mouth Disease Virus) combined vaccine. All calves were boosted subcutanously
with Rabicine vaccine 11.3 months later.
The results of this experiment are shown in Figures 5 and 6. Figure 5 depicts the
scatter of the immune response of the calves in the three groups around the average
neutralization titer, while Figure 6 shows the percentage of calves that developed
antibodies as a function of the route of vaccination. As can be seen there are no
significant differences between the three groups. The percentage responders and the
titer are usually higher close to vaccination time. The booster vaccination had a
significant effect on both the numbers of animals responding and the neutralization
titer. A comparison between NTCEIA and ELISA tests is shown in Figure 7. The total
number of positive calves in NTCEIA is compared to the number positive in ELISA.
There is a general agreement between the two tests but in most cases ELISA reveal a
lower number of positive calves. This might be because of the lower sensitivity of the
ELISA or possibly that each test identifies a different class of antibodies, which differ
in titer and time of appearance.
In another survey 7 cows from farm ED were tested for neutralizing antibodies
following subcutaneous vaccination with Rabicine vaccine. The cows were boosted
11.5 months later. At different times following vaccination the sera were tested by
NTCEIA. The results shown in figures 8 and 9 are similar to those obtained with the
calves. The booster vaccination affected both the number of responders and their
neutralizing antibody titers.
Discussion
The rapid fluorescence foci inhibition test (RFFIT) which was developed as an
alternative to the classic mouse neutralization test (MNT) is not suitable for field
serosurveys where many sera are to be tested. It requires many hours of microscopic
observation and the interpretation of the results, in many cases, are subjective and
cannot be quantitated accurately. Furthermore, when the same sera were tested by
RFFIT in several laboratories, large discrepancies in the results were obtained. A new
method was developed to render this unique test for rabies to be more reliable and
similar to neutralization tests developed for other viruses (10), but the new test FAVN (Fluorescent Antibody Virus Neutralization (10)) is also unsuitable for largescale serosurveys. To overcome this problem we have developed the NTCEIA, a
tissue culture neutralization test in which the results are read automatically by an
ELISA reader. The dilutions of the sera and virus are done directly in the wells and
the cells are seeded into the same wells, using ELISA multipipettes. All the necessary
controls are run in the same plates so if the format of the test is uniform the results can
be automatically analyzed by computer. This test can therefore be more rapid than
RFFIT or similar assays and the format allows the screening of 12 sera in one plate.
Similar tests were developed in several laboratories for a variety of viruses (5-8), and
all used the horseradish peroxidase - antibody conjugates. We found that the alkaline
phosphatase conjugates performed much better with cattle serum and that to avoid
high background reading the test has to be adapted specifically for each animal
species.
The function of the neutralization test is to establish the immune status of the
vaccinated human or animal. For humans, 0.5 IU is accepted as a minimal protective
titer. In our test, we established that 1 IU is equal to 28 NTCEIA units and 0.5 IU,
therefore, equals 14 NTCEIA units. The NTCEIA was reproducible (coefficient of
variation (CV) = 20%) and is in good agreement with the MNT.
The test has another advantage as it can also serve as a rapid titration of the virus. We
have found that with NSKSH cells and 48h incubation 5 MICLD50 of rabies virus can
easily be detected..
In the past an ELISA for cattle serum, was developed to measure vaccination efficacy
in Israeli herds (3). However, the correlation of this test with MNT was poor and did
not allow an accurate estimate of the immune status of individual animals or herds.
We therefore adapted the NTCEIA test for cattle sera and using our standard cattle
serum, we demonstrated the reproducibility of the test (CV = 17% for 17
measurements) and the ease with which serosurveys can be conducted.
Assuming that the protective neutralizing antibody levels are the same for humans and
cattle, 14 NTCEIA units or more in cattle sera (CNTU) indicate a state of immunity
against rabies. The standard cattle serum was used for a small-scale study performed
in two farms (M for calves and ED for cows) designed to establish the efficacy of
different routes of anti-rabies vaccination of cattle (Figures 5,6, 8 and 9). Calves and
cows responded satisfactory to the Rabicine vaccine within two weeks following
vaccination and the route of vaccination had no significant effect on neutralizing
antibody levels. Booster vaccination significantly increased both neutralizing
antibody titer and the percentage of responders. When compared to ELISA, NTCEIA
was found to be more sensitive (Figure 7).
In tests performed with 45 bovine sera a good correlation was obtained between
NTCEIA and the standard mouse neutralization test (Table 2). These results
strengthen the usefulness of NTCEIA for large scale rapid neutralization.
A further advantage of the test is that it can be easily adapted to screen neutralizing
antibodies in other animals species. In another paper (11) we report the application of
the test to study
the efficacy of oral vaccination of foxes, jackals and rodents with a vaccinia-rabies
recombinant virus vaccine.
* Supported by a grant from the Israeli Dairy Board, to D.K. and P.F.
References
1. Pastoret, P.P., Brochier, B., Blancou, J., Artois, M., Aubert, M., Kieny, M.P.,
Lecocq, J.P., Languet, B., Chappuis, G. and Desmettre, P.: Development and
deliberate release of vaccinia-rabies recombinant virus for the oral vaccination of
foxes against rabies. In: Recombinant Poxviruses. ( Binns, M. M. and Smith, G. L.
eds.) pp. 163. CRC Press Inc., Boca Raton, Ann Arbor, London, Tokio,1992.
2. Pastoret, P. P., Brochier, B. and Coppens, P.: Deliberate release of a recombinant
vaccinia-rabies virus for vaccination of wild animals agaist rabies. Microbiol.
Releases 1:191-195, 1993.
3. Katz, D., Freeman, E., Davidson, M., Abramson, M., Galan, O. and Fuchs, P.:
ELISA for evaluation of anti-rabies vaccination efficacy in cattle. VII Intern. Con.
Virol., Edmonton, Canada. R23.67, p. 182, 1987.
4. Shapira, A., Lustig, S. and Katz, D.: Neutralization of Sindbis virus in tissue
culture by a radioimmunoassay. Isr. J. Med. Sci. 16:473, 1980.
5. van Tiel, F. H., Harmsen, T., Wagenaar, M., Boere, W.A.M., Kraaijeveld, C.A.
and Snippe, H.: Rapid determination of neutralizing antibodies to Semliki Forest
virus in cell cultures with virus specific monoclonal antibodies. J. Clin. Microbiol.
24:665-668, 1986.
6. Anderson, L.J., Hierholzer, J.C., Bingham, P.G. and Stone, Y.O.:
Microneutralization test for Respiratory Syncytial virus based on an enzyme
innunoassay. J. Clin. Microbiol. 22:1050-1052, 1985.
7. Grom, J. and Bernard, S.: Virus enzyme-linked cell immunoassay (VELCIA):
Detection and titration of Rotavirus antigen and demonstration of Rotavirus
neutralizing and total antibodies. J. Virol. Methods. 10:135-144, 1985.
8. Mannen, K., Mifune, K., Reid-Sanden, F. L., Smith, J. S., Yager, P. A., Sumner, J.
W., Fishbein, D. B., Tong, T. C. and Baer, G. M.: Microneutralization test for rabies
virus based on an enzyme immunoassay. J. Clin. Microbiol. 25:2440-2442, 1987.
9. Atanasiu, P.: Quantitative assay and potency test of antirabies serum and
immunoglobulin. In: Laboratory Techniques in Rabies, 3rd edn. (Kaplan, M. M. and
Koprowski, H. Eds.) pp. 314-318. WHO, Geneva, 1973.
10. Cliquet, F., Aubert, M. and Sagne, L.: Development of a fluorescent antibody
virus neutralization test (FAVN test) for the quantitation of rabies-neutralizing
antibodies. J. Immunol. Meth. 212: 79-87, 1998.
11. Fuchs, P., King, R., Zamir, S., Freeman, E., Davidson, M., Aubert, M., and Katz
D.,: Detection of anti-rabies neutralizing antibodies by tissue culture enzyme
immunoassay (NTCEIA) in rodents and canines after oral vaccination with a
vaccinia-rabies recombinent vaccine, Israel J. Vet. Med. 53:143-153, 1998.
ISRAEL JOU
ISRAEL JOURNAL OF VETERINARY MEDICINE
Figure 5: Neutralizing anti-rabies antibodies in calves at different times after vaccination by different
routes as determined by NTCEIA.
All calves received a booster vaccination 11.3 months after the first vaccination.
Legends to Figures
Figure 1: TCEIA for rabies virus titration in two types of microplates. Comparison between BHK-21
and SKNSH cells and between two types of conjugates.
A: Costar microplates
B: Nunc microplates
SKNSH cells
BHK-21 cells
GAR-AP Goat anti-rabbit alkaline phosphatase conjugate
PA-AP Protein A alkaline phosphatase conjugate
n Rabbit anti-rabies serum
s Normal rabbit serum
n Cattle Standard Serum
q International Human Rabies
Immunoglobulin Preparation
Figure 2: Titration of rabies virus in SKNSH cells by TCEIA.
Figure 3: Titration of anti-rabies neutralizing antibodies of the International human Rabies
immunoglobulin preparation and the Cattle Standard Serum.
n % Neutralization from experimental data
q % Neutralization calculated from regression analysis
Figure 4: Determination of NTCEIA neutralization titer of the Cattle Standard Serum.
B: Nine calves vaccinated
intramuscularly
A: Nine calves vaccinated
subcutanously
C: Ten calves vaccinated
subcutanously with
Rabicine+FMDV
vaccine
Antibody response determined by NTCEIA
Antibody response determined by ELISA
Figure 6: The effect of the route of vaccination of calves on the anti-rabies neutralizing antibody
response as measured by NTCEIA.
Subcutaneous vaccination
Intramuscular vaccination
Subcutaneous vaccination with Rabicin+FMDV
Figure 9: Percent of cows responding to vaccination as determined by NTCEIA.
Booster injection was given 11.5 months after the first vaccination.
Figure 7: Comparison between anti-rabies antibody titers in calves measured by ELISA and NTCEIA.
Figure 8: Neutralizing anti-rabies antibodies in seven cows at different times after vaccination.
Booster injection was given 11.5 months after the first vaccination.