Cellular-Signaling-Pathways
advertisement

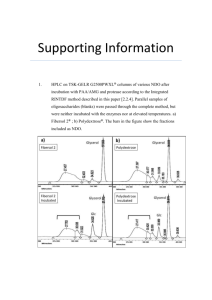
Cellular signaling pathways affected in neurofibromatosis The NF1 gene product neurofibromin is a tumor suppressor protein that suppresses Ras function. The NF2 gene product Merlin (a.k.a. schwannomin) is a tumor suppressor protein that suppresses PAK1 function. Below is a simplified diagram showing some of the cellular signaling pathways involved in neurofibromin and merlin function. These signaling pathways also represent some of the candidate therapeutic targets for the treatment of NF1 and NF2, in which the tumor suppressor functions of neurofibromin and merlin, respectively, are disabled. The ‘downstream signaling pathways’ are progressively becoming a focus as effective, specific therapeutics for NF1 and NF2 are sought. Cytokine receptors EGFR, PDGFR, etc. SOS, Grb2 Ras Merlin (NF2) Rac1,2 PI3 K AKT PAK1 Rho mTOR Neurofibromin (NF1) C-Raf MEK 1,2 ERK 1,2 ……………………….Downstream Signaling Pathways……………………………….. © 2006 KHS/Children’s Tumor Foundation Drugs in recent, current or planned (*) testing for NF DRUG Pirfenidone DISORDER NF1 Iressa (gefitinib) Gleevec (imatinib) Zarnestra (Tipifarnib) NF1/NF2 INDICATION Plexiform neurofibroma Meningioma NF1/NF2 Meningioma Rapamycin PEG-INTRON (pegylated interferon alpha) CELL TARGET Fibrosis PHASE III EGFR II PDGFR; BcrAbl, KIT/PI3 Plexiform Farnesyl protein neurofibroma transferase II NF1 NF1 MPNST Plexiform neurofibroma mTOR Jak-Stat II* I Erlotinib Lovastatin NF1 NF1 MPNST Cognitive function EGFR HMG CoA Reductase I I Simvastatin NF1 Cognitive function HMG CoA Reductase I Nexavar (Sorafenib) NF1 Plexiform neurofibroma Raf Kinase I* NF1 II For fully updated information on current NF clinical trials please visit: http://www.ctf.org/research/ © 2006 KHS/Children’s Tumor Foundation Other drugs in development that target NF-relevant pathways Below is a partial list of drugs that are currently in clinical testing for disorders other than NF; but which may also be relevant to NF. This list is provided for the purposes of highlighting examples (but is not all-inclusive) of some of the potentially relevant signaling pathway targets for NF therapeutics. This information was compiled from public resources including www.PhRMA.org) Drug Candidate Signal Transduction target Avastin (Bevacizumab) VEGF Erbitux (Cetuximab) EGFR In current testing for cancer indications… Various Most advanced status is … Marketed Head and neck; colorectal; various Marketed Sutent (Sunitinib) PDGFR/VEGFR/c-Kit/ GIST. Kidney, breast, lung; enal cell FLT-3 carcinoma; stomach cancer, liver, breast, prostate Velcade (Bortezomib) Proteasome Multiple myeloma; lung Marketed Marketed Certican (Everolimus) FKBP-12/mTOR Breast; various Phase 3 Depsipeptide (FK228) HDAC/PAK-1 Phase 3 Enzastaurin HCl (LY317615l) Lapatinib PKC-beta Cutaneous T-cell lymphoma, renal cell carcinoma, hormone refractory prostate cancer Glioblastoma; various EGFR/ErbB2 Phase 3 Panitumumab EGFR Breast, head/neck, kidney, bladder, lung, stomach. Metastatic colorectal; kidney; lung. Sarasar (Lonafarnib) Farnesyl protein transferase (FPT) Gliomas; head and neck; breast; CMML; myelodysplastic syndrome Phase 3 Temsirolimus HGF/Met/FKBP12/ mTOR Metastatic breast cancer; lymphoma; kidney cancer Phase 3 Vatalanib Metastatic colorectal Phase 3 AEE788 AP23573 multi-VEGFR/ PDGFR/c-kit VEGFR/ErbB2 mTOR Glioblastoma multiforme; various Glioblastoma; various Phase 2 Phase 2 BMS-354825 Src-Abl CML; various Phase 2 CI-1040 (PD184352) MEK1/2; ERK Lung, breast, colon, cancer Phase 2 CP-547,632 VEGFR Phase 2 iCo-007 c-raf Ovarian, peritoneal, fallopian tube cancer Not cancer - AMD/ocular diseases INSM-120-101 (rhIGFBP-3) MDX-214 IGFR/Erk Phase II for mytotonic dystrophy. Cancer safety trials ongoing. Various Phase 2 AML; mast cell leukemia Phase 2 Various Phase 2 lung Phase 2 EGFR Midostaurin (PKC412) VEGFR/PDGFR/PKC/ c-Kit Perifosine (KRX-0401) Akt/JNK/MAPK SAI-EGF EGFR Phase 3 Phase 3 Phase 2 Phase 2 Zactima (ZD6474) EGFR lung Phase 2 17-AAG B-Raf/HSP90 Von Hippel Lindau; various Phase 2 AVE0005 VEGF Safety - cancer Phase 1 BMS-273291 VEGFR2 Safety - cancer Phase 1 LErafAON - ETU Raf-1 Safety - cancer Phase 1 © 2006 KHS/Children’s Tumor Foundation