Fractional Distillation Lab
advertisement

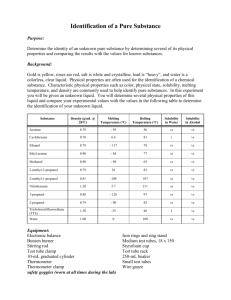
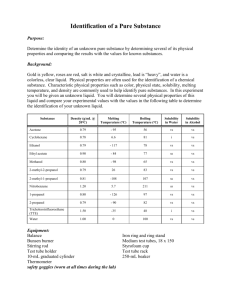
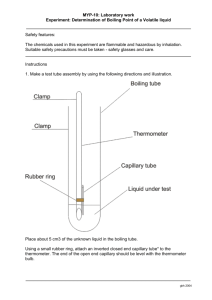
Page 1 NAME________________________________ PERIOD____ FRACTIONAL DISTILLATION LAB This lab will take a number of days to complete. During this time we will be finding the properties of a liquid mixture, separating the mixture, and finally identifying the parts which make up the mixture. Each day you will be given the objective, apparatus, and procedures for that class period. Answer the following questions before you begin the lab. 1. A special property is __________________________________________ 2. The objective of the fractional distillation lab is to __________________ and _______________________liquids that are dissolved together. 3. Definition of Fractional Distillation - Fill in the blanks Fractional distillation is the process of separating two or more soluble liquids by _________________ and changing the liquid with the lower ______________ to a _____________________ which is called _______________________. The gas then travels to the other test tube which is cooled and changes the gas back to a ______________ which is called _____________________ 4. How can we prove if we were successful in separating the liquids? 5. List the special properties we use to identify liquids ________________________ _________________________ _________________________ _________________________ __________________________ 6. Write the procedures for the following tests as demonstrated in class a. Flammability ______________________________________________________________________ ______________________________________________________________________ b. Solubility of Sugar ______________________________________________________________________ ______________________________________________________________________ c. Odor ______________________________________________________________________ ______________________________________________________________________ 106742490 1 Page 2 d. Boiling Point – see procedures in Day 1 of the lab e. Density (the mass of 1 ml of liquid) – Remember the more volume in the 10 ml graduated cylinder – the more accurate. a. b. c. d. e. How do you find the volume of liquid? ______________________________ Which graduated cylinder is more accurate? __________________________ Explain your answer for letter B. _______________________________ How do you find the mass of a liquid? ______________________________ What is the formula for density? _______________________________ 8. Before you begin the lab you will need to read through the packet, define the words below, and do the density review on the next page 1. special (characteristic)property __________________________________________________________________ ___________________________________________________________________ 2.distillation -_________________________________________________________ ___________________________________________________________________ 3. fractional distillation -_________________________________________________ ___________________________________________________________________ 4. condensation -______________________________________________________ ___________________________________________________________________ 5. vaporization -_______________________________________________________ ___________________________________________________________________ 6. condensing tube -____________________________________________________ ___________________________________________________________________ 7. distilling tube -______________________________________________________ ___________________________________________________________________ 8. flammability -_______________________________________________________ ___________________________________________________________________ 9. density -___________________________________________________________ ___________________________________________________________________ 10. solubility -________________________________________________________ ___________________________________________________________________ 11. boiling chips -______________________________________________________ ___________________________________________________________________ 12. fraction -___part of a whole___________________________________ ____________________________________________________________________ 13. plateau -___________________________________________________________ _____________________________________________________________________ 14. boiling point -________________________________________________________ _____________________________________________________________________ 15. tip of the probe -__where the temperature is measured______________ _____________________________________________________________________ 106742490 2 Page 3 DENSITY REVIEW Read the 10 ml graduated cylinders 2ml 2 ml 1 ml 2 ml 1 ml 1ml 0 ml Find the density of the liquids below. Be sure that you; a. write the formula b. substitute the number and units c. write the answer with the correct units 1. Mass of the empty graduated cylinder = 6.9 g Mass of grad. cylinder plus liquid = 16.9 g volume of liquid = 5 ml DENSITY = ____________ 2. Mass of empty graduated cylinder = 28.1 g Mass of graduated cylinder and 8 ml of liquid = 35.2 g DENSITY = ___________________________ 3. mass of graduated cylinder = 28.2 g mass of graduated cylinder and 6.2 ml = 38.1 g DENSITY = __________________________ 4. mass of graduated cylinder = 28.4 g mass of graduated cylinder and 2.3 ml = 30.7 g DENSITY = _________________________ 5. Find the density of the liquid from the data in the pictures 3 ml 7.1 g DENSITY = ____________ 106742490 13.4 g 3 Page 4 DAY 1 - PROPERTIES OF THE WHOLE SOLUTION OBJECTIVE - To find the properties of the whole mixture of liquids APPARATUS test tubes balance bunsen burner wood splint graduated cylinder distilling apparatus sugar boiling chips beaker ring stand PROCEDURES 1. Place 5 ml of the sample into two different test tubes. 2. One lab partner will be working on procedure numbers 3 through 6 and the other partner will be working on procedure number 7. You will be sharing your data with your partner after you complete today’s lab. 3. Check for flammability (does the substance burn) of the liquid with a wood splint 4. Make observations of the odor (smell) 5. Find the density (the mass per ml) 6. Check the solubility of sugar (will it dissolve) 7. Find the boiling point of the liquid (the temperature at which the liquid changes to a gas) DRAW THE SETUP HERE Procedures to find boiling point 1. Place a 5 ml sample in the large test tube (distilling tube – the test tube that is heating and vaporization occurs) 2. Add several boiling chips (rocks which allows the liquid to heat evenly) 3. Place the distilling apparatus (the thermometer, stopper, and hose) in the large test tube. 4. Put 500 ml of water into the 600 ml beaker 5. Place the end of the hose in a small test tube (condensation tube – the test tube where the vapor cools and condensation occurs) which is in the 600 ml beaker. 106742490 4 Page 5 6. Heat SLOWLY with the Bunsen burner (rate should about 5 degrees per minute) until almost dryness Reminder on how to control the heating: 1. Move the ring stand before starting 2. Leave space between the test tube and Bunsen burner. 3. Turn down the Bunsen burner with the silver valve. 7. Record the temperature every 30 seconds – DURING THE LAB. 8. After checking that each of you has all the data BOTH of you must graph the boiling point of the liquid and answer the questions. DATA SHEET FLAMMABILITY _________________ ODOR__________________________ DENSITY MASS OF GRAD. CYLINDER _____ MASS OF G. C. AND LIQUID _____ MASS OF LIQUID ______________ VOLUME OF LIQUID __________ DENSITY____________________ BOILING POINT (include you data table with time and temp.) ______________ SOLUBILITY OF SUGAR _______ 106742490 5 Page 6 QUESTIONS 1. At what temperature does your graph have plateaus (level part of the graph) ______________________________________________________________________ ______________________________________________________________________ 2. What do you think is happening at the plateau? Explain your answer. ______________________________________________________________________ ______________________________________________________________________ 3. How many fractions (parts) of mixture should you collect day 2 of the lab? Explain your answer. 4. Show on the graph below where you would switch test tubes to collect the different liquids from fractional distillation (separation of liquids with different boiling points). Why? t e m p (C) time (min) 5. In the above question, how will you know when to switch test tubes based on your thermometer reading? ______________________________________________________________________ ______________________________________________________________________ 106742490 6 Page 7 Use this graph to answer the following questions boiling point of a mixture of liquids temp (celsius) 100 80 60 40 20 0 2 4 6 8 10 12 14 16 18 20 22 time( min) 1. What are the boiling points of these liquids? _______________________ 2. Is the mixture boiling between 0 and 1.5 minutes? _______________________ 3. What time does the liquid first start to boil? _______________________ 4. What time does the second liquid start to boil? _____________________ 5. If you collected the vapor during the first plateau, would you have a pure liquid? If not, what would the substance be? _____________________________________ 6. If you collected the vapor during the second plateau, would you have a pure liquid? If not, what would the substance be? ______________________________ 7. If you collected the vapor between plateaus, would your have a pure liquid? If not, what would the substance be? _____________________________________ 8. According to the graph, during what time period would you collect your first pure liquid? _______________________ 9. According to the graph, during what time period would you collect your second pure liquid? _______________________ 106742490 7 Page 8 DAY 2 - SEPARATING THE MIXTURE OBJECTIVE - to use fractional distillation to separate the mixture of liquids APPARATUS distilling apparatus 3 small test tubes boiling chips # 2 stoppers burner ring stand one large test tube masking tape 600 ml beaker clamp burner screen PROCEDURE 1. Pour 25 ml of the solution into a large test tube (distilling tube - test tube which has the liquid to be separated.) 2. Add 3 or 4 boiling chips 3. Place the distilling apparatus (the thermometer, stopper, and hose) in the large test tube. 4. Put 500 ml of water into the 600 ml beaker 5. Place the end of the hose in the condensing tube which is in the 600 ml beaker. REMINDERS a. Keep the rubber hose below the water level of the beaker but not in the liquid b. Do not heat so fast that the liquid jumps to the condensing tube. Your liquid should not be any shade of the liquid that you are distilling. c. You may heat faster once you have reached 100 0C. d. Record temperature every minute – Put a dash when the mercury reading is behind the black stopper. 6. Switch you test tubes a. After the first plateau b. At the beginning of the second plateau - when the temperature starts to stay the same. You can stop when you have enough liquid to test. c. Mark on your data table when you switched test tubes fraction 1 fraction 2 fraction 3 label and save trash label and save 5. Label fraction 1 and 3 with the following information fraction # period names of group members 106742490 8 Page 9 DAY 3 – SPECIAL PROPERTIES OF THE FRACTIONS OBJECTIVE - to use the special properties to identify the fractions (parts) APPARATUS balance test tubes boiling chips fraction 1 and 3 burner ring stand burner screen distilling apparatus wood splint sugar PROCEDURES 1. Find the flammability of each of the fractions 2. Find the odor of each of the fractions 3. Find the density of each of the fractions 4. Find the boiling point of each of the fractions a. The set up is just like day 2 b. Record temp every 30 seconds c. Stop when you have a plateau for 6 times. Keep the temperature under 1030 C. 5. Do the solubility of sugar last - Sugar will throw off your other tests. 6. Graph your data on a multiple line graph. DATA SHEET TEST FRACTION 1 FRACTION 3 FLAMMABILITY ______________________________________________________________________ ODOR DENSITY MASS OF GRAD. CYLINDER ____________ ____________ MASS OF G. C. AND LIQUID ____________ ____________ MASS OF LIQUID ____________ ____________ VOLUME OF LIQUID ____________ ____________ DENSITY ____________ ____________ ______________________________________________________________________ BOILING POINT ______________________________________________________________________ SOLUBILITY OF SUGAR ______________________________________________________________________ 106742490 9 Page 10 GENERAL CONCLUSION QUESTIONS 1. The process of separating mixtures of liquids by boiling them and collecting the liquids in different test tubes is called ___________________. 2. Two liquids can be separated if they have different ________________________. 3. You have a mixture of 3 liquids. One boils at 56 0C, the second boils at 79 0C, and the third boils at 100 0C. a. Sketch a boiling point graph for the mixture and label the temperature of the plateaus T e m p 0 C Time (min) b. Draw a boiling point graph for each liquid and label the boiling points T E M P 0 C T E M P 0 C T E M P 0 C Time (min) Time (min) Time (min) c. How many test tubes of pure liquid would you have collected? __________ d. Mark on graph in question 3a with an X where you would have switched the condensing tubes. 4. What is the purpose of the beaker of cold water? ________________________________________________________________________ ________________________________________________________________________ 106742490 10 Page 11 5. When the thermometer bulb (the part of the thermometer where the mercury is stored and the point where the temp is measured) is at the top of the distilling tube, what is it measuring? ________________________________________________________________________ ________________________________________________________________________ 6. When the thermometer bulb is at the bottom of the distilling tube, what is it measuring? ________________________________________________________________________ ________________________________________________________________________ Word bank – the words may be used more than once or not at all Boiling point Begins to rise Constant temperature Lowest boiling point Pure two plateau condensing tube three nothing alcohol water trash second plateau higher thermometer collecting Liquids separate when heating because they have different ____________________. The condensing tube collects _________________ during the beginning until the mixture reaches the ________________________________. Fraction # 1 is ______________. We switch the ___________________ after the _________________ ends. This will separate the _____________________ from the trash. We know to make the first switch when the thermometer _____________________________. We continue ______________fraction # ___________ in the _________________ until the graph reaches the __________________________. We know we reached the ____________________ because the ________________________ is staying at a ______________________. After several of the same temperature readings we switch the ____________________. Now we are collecting the # ________ fraction and it will be ______________. It is the liquid with the ___________________ boiling point. Fraction number 3 is ________________ 106742490 11 Page 12 Fill in the chart using the data your group collected. Density Boiling point Flammability Odor Solubility of sugar Alcohol Water .79 g/ml 82.5 C Yes Strong No 1 g/ml 100 C No None Yes Whole solution Day 1 Fraction #1 Fraction #3 Write a conclusion for the lab. The conclusion should include the following items and be in paragraph form. Good copies!!!!!!!!!!! A 20 point conclusion includes: o State your hypothesis as to how many liquids you had in your solution -day 1 should be referenced. o Review and evaluate your separation results and techniques. Support with data from day 3. o Identify the fractions – Support with data more is better then less for solid proof. o Evaluate the purity of each of the fraction- How close your experimental results to the theoretical were listed. o How would you modify your procedures next time to improve your success? What would you change now that you have done this separation once for the next time you do this during sludge? Grades of 18, 16, 14, 12, 10 will be given for conclusion not including all aspects of this typical format. 106742490 12