This form, requesting approval for changes to the Inventory of X
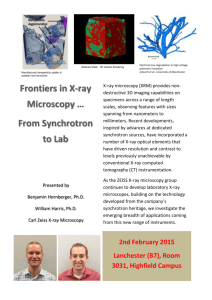
advertisement

SCHEDULE 2 AMENDMENT APPLICATION FORM – X-RAY EQUIPMENT This form, requesting approval for changes to the Inventory of X-ray Equipment in Schedule 2 of an existing licence, must be completed and forwarded to the EPA’s Office of Radiological Protection at least 28 days in advance of the date of the proposed changes Licence No: __________________________________________________________________________________________________ Licensee Name: Type of Amendment (please tick appropriate box and complete relevant sections) If the amendment is a replacement of an existing source please tick both boxes 1 and 2 and complete amendment type 1 and 2. 1. Addition of a X-Ray Unit to Licence 2. Removal of an Existing X-Ray Unit from Licence 3. Change in Practice or Licensing Exception for Existing X-Ray Unit Please specify and justify the reason why this licence amendment is sought [Please type here] Office Use Only Administration Date P/W Rec: ________ Date P/W Checked: ___________ P/W Correct: Y/N Initials: _____ Inspectorate P/W Approved: Y/N Initials: _____ Notes: Page 1 of 4 AMENDMENT TYPE 1 (Addition of an X-ray Unit to Schedule 2) Please note this amendment will initially be for “Custody and Commissioning Purposes only”. The X-ray equipment shall not be acquired until this amendment has been approved. This amendment request must be accompanied by: 1) Revised Risk Assessment (where relevant) 2) Revised Radiation Safety Procedures (where relevant) Where this is a replacement of an existing X-ray unit, the existing risk assessment must be reviewed to determine if there are any revisions required to the radiation safety procedures. Where applicable, the revised risk assessment and revised radiation safety procedures must accompany the amendment. (Note: The radiation safety procedures for Dental and Veterinary Practices are contained in the relevant RPII Codes of Practice 1) In the case of medical and dental X-ray units, the licensing exception ‘Custody and Commissioning Purposes’ will be removed once a copy of the commissioning report has been received. This report must include a statement from the Radiation Protection Adviser (RPA) that the X-ray unit is fit for use. For all other X-ray units the licensing exception ‘Custody and Commissioning Purposes’ will be removed once a copy of the installation report has been received. Name and address of Supplier: Manufacturer: __________________________________________________________________ _______________________________________________________________________________ Model: _______________________________________________________________________________________ Serial Number/unique Identifier of X-ray tube: ______________________________________________________ Number of X-ray tubes per machine: _______________________________________________________________ X-ray tube kilovoltage (Max kVp): X-ray tube Current (Max mA): _________________________________________________________________ ____________________________________________________________________ Purpose (e.g Radiography, Fluroscopy, Security Screening, etc): _______________________________________ Type of X-ray Unit (Fixed/Mobile/Handheld): ________________________________________________________ For Dental X-ray equipment please specify the unit type (intra oral/OPG/Cone Beam CT/hand held): _________ For Veterinary X-ray equipment please indicate whether the unit is: Portable unit in fixed location Portable unit used in the field outside surgery State the name of the Area/Location/Room where X-ray unit will be used/stored (this will appear on Schedule 2): ___________________________________________________________________________________________ Proposed Date of Installation (following EPA Approval): _______________________________________________ 1 Code of Practice for Radiological Protection in Dentistry, Radiological Protection Institute of Ireland, 1996. Code of Practice for Radiation Protection in Veterinary Medicine, Radiological Protection Institute of Ireland, 2002. Page 2 of 4 AMENDMENT TYPE 2 (Removal of X-ray Unit from Schedule 2) Please note this amendment will initially be ‘For disposal’ Please note that X-Ray equipment that has been rendered incapable of producing ionising radiation must be disposed of in accordance with the requirements of the WEEE regulations. For guidance on disposal, please refer to the EPA/RPII Guidance Note on the Management of X-ray Units at End-of-Life, January 2011 (Revision) which may be downloaded from the EPA website (www.epa.ie). The licensing exception ‘For Disposal’ will be removed once confirmation and documentary evidence has been provided to the EPA that the X-ray unit has been removed from the licensee’s premises or rendered inoperable. Manufacturer: _______________________________________________________________________________ Model: _______________________________________________________________________________________ Serial Number/unique Identifier of X-ray tube: ______________________________________________________ Please state method and date of removal of the X-ray Unit, e.g. disposal, transfer to a third party, selling to another licensee, rendered inoperable, etc : ____________________________________________________________________________________________ If transferring X-ray unit to another licensee please give their name and address: ____________________________________________________________________________________________ __ ____________________________________________________________________________________________ AMENDMENT TYPE 3 (Change in Practice or licensing exception for existing X-ray Equipment)Please complete this section when there is a request to change the licensing exception listed on Schedule 2 of the licence for the X-ray Unit or if there is a request to change the practice listed on the front cover of the licence. Manufacturer: _______________________________________________________________________________ Model: _______________________________________________________________________________________ Serial Number/unique Identifier of X-ray tube: ______________________________________________________ Purpose of X-ray tube as specified on Schedule 2 of the Licence: _______________________________________ Location of Source as specified on Schedule 2 of the Licence: _________________________________________ Change in Practice (e.g. removal of use from licence cover): ___________________________________________ _ _____________________________________________________________________________________________ Change in licence exception (e.g. removal of “Custody and Commissioning Purposes”) _ _____________________________________________________________________________________________ Page 3 of 4 I hereby apply for a Schedule 2 amendment to the above licence. I declare that to the best of my knowledge, the particulars given above are true. Signed: _______________________________________________________________________________________ Name (print or type): ____________________________________________________________________________ Position: Date: ______________________________________ This Amendment Application Form must be signed by the Managing Director/Chief Executive, Hospital Manager (or equivalent member of senior management), Radiation Protection Adviser (RPA) or Radiation Protection Officer (RPO) nominated by the licensee submitting the Application. In the case of a licence issued in the name of an individual person the form must be signed by that person. On completion, this Amendment Application Form should be sent to: Radiation Protection Regulation Environmental Protection Agency Office of Radiological Protection 3 Clonskeagh Square Dublin 14 Telephone: Fax: Website: (01) 2680100 (01) 2680199 www.epa.ie Note: The form and any supporting documents may be scanned as a pdf and sent by email to radregulatory@epa.ie. Version: August 2014 Page 4 of 4