Separating a Mixture Lab:
advertisement

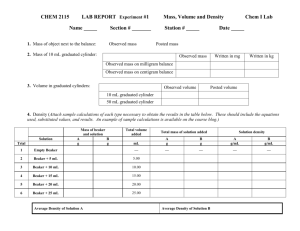
Name: __________________________ Date: ____________ Period: _____ Lab Station: _____ Separating a Mixture Lab: A Quantitative Analysis OBJECTIVES/PURPOSE: Use a variety of methods to separate the components of a mixture. To demonstrate the law of conservation of mass. PRE-LAB QUESTIONS: 1. Define matter. Give an example of something that is matter, and something that is not matter. 2. Compare and contrast pure and not pure substances. Give two examples of each. 3. How are elements and compounds similar? How are they different? 4. How are compounds and mixtures similar? How are they different? 5. How are homogeneous mixtures different from heterogeneous mixtures? 6. Is a solution of salt water a homogeneous mixture or a heterogeneous mixture? How do you know? 7. State the law of conservation of matter. 8. Create a data chart to record your quantitative and qualitative observations. MATERIALS: Beaker, 50ml (2) Beaker, 250ml (1) Bunsen burner with matches Filter paper (write your group’s name on it) Glass funnel Iron, salt, sulfur mixture (1 bag per class) Magnet in a plastic bag Weigh boat (throw away at end of lab) Beaker tongs Ring stand with ring clamp Stirring rod Distilled water Wire gauze Electronic balance Sharpie (to label filter paper) Scoopula Watch glass Clay Triangle Always wear safety goggles to protect your eyes. If you get a chemical in your eyes, immediately flush the chemical out at the eyewash station while calling to your teacher. Know the location of the emergency lab shower and eyewash station and the procedures for using them. PROCEDURES: Part I: 1. Find the mass of an empty weigh boat 2. Using a scupula, place a 10 gram mixture of iron, sulfur, and salt on a weigh boat. Find the mass. 3. Take the mass of an empty 50-ml beaker. 4. Remove the iron from the mixture with the aid of a magnet. 5. Transfer the iron to the 50-ml beaker. 6. Take the mass of the beaker with the iron in it. 7. Calculate the mass of just the iron. Name: __________________________ Date: ____________ Period: _____ Lab Station: _____ Part II: 8. Carefully, transfer the sulfur/salt mixture that remains to a second 50-ml beaker. Add about 15-ml of distilled water and stir with a glass stirring rod to dissolve the salt. 9. Take the mass of the 250-ml beaker. 10. Using a Sharpie marker, write your names or initials along the perimeter of the filter paper. 11. Take the mass of the filter paper. 12. Fold it in half and then in half again. Place the filter paper in the funnel. Place the end of the funnel into the 250-ml beaker. (Using a clay triangle can help hold a short funnel in place.) Filter the mixture, and collect the filtrate (the liquid that passes through the filter). 13. The 50-ml beaker probably still has a sulfur and salt sludge in it. Use the glass rod to scoop any excess out. Then, pour another 15 ml of distilled water into this beaker and filter it again. If necessary, do this a third or fourth (but not more than a sixth) time to filter as much sulfur and salt water as possible. 14. Carefully, remove the filter paper from the funnel, unfold it, and set it out to dry overnight in the designated location. Watch your fingers. 15. Wash any residue in the funnel with 15-ml of distilled water, and collect the rinse water with the filtrate in the 250-ml beaker. Don’t lose any of the mixture! Part III: 16. Take the mass of the clean watch glass. 17. Set up a ring stand and a Bunsen burner so that the clamp is about six inches above the Bunsen burner. Place wire gauze atop the ring clamp. 18. Place the watch glass so that it sits atop the beaker, and use beaker tongs to place the 250ml beaker atop the wire gauze. Make sure you have beaker tongs. 19. Ask your instructor for permission to light the Bunsen burner. Evaporate the water from the filtrate. Warning: If you have more than 75ml of water in the beaker, the water may boil over; if the flame is too far away, the water won’t boil quickly enough. Clean up the equipment you’ve finished using – but not the beaker tongs. Make certain someone is monitoring your flame at all times! 20. The weigh boat should be thrown away. Return the iron to the beaker under the hood. Part IV: 21. Stop heating just before the liquid completely disappears; you’ll hear a crackling sound. After the water has evaporated, turn off the gas at the sink. 22. Use beaker tongs to carefully remove the beaker. Place it on the lab counter, and let it cool. 23. After the beaker has cooled, carefully remove the watch glass, and take the mass of the beaker with the salt in it. 24. What is the mass of just the salt that has accumulated in the beaker? 25. Now, take the mass of the used watch glass. 26. What is the mass of just the salt that has accumulated on the watch glass? 27. What is the total mass of the salt accumulated in the beaker and on the watch glass? 28. Thoroughly wash and dry your supplies and lab station. Part V: Day 2 29. What is the mass of the dried filter paper and sulfur? 30. What is the mass of just the sulfur? 31. Dispose of the sulfur and filter paper in the trashcan. Name: __________________________ Date: ____________ Period: _____ Lab Station: _____ Separating a Mixture Lab Analysis Questions 1. What properties did you observe in each of the components in the mixture, and how did those properties help you to separate the components of the mixture? 2. What is distilled water? How is it different from tap water? Why is it important to use distilled water in this experiment? 3. Was adding 15 mL of distilled water to our mixture a chemical or a physical change? How do you know? 4. Why could we add water without jeopardizing our results? Why wasn’t it important to be accurate or precise with our water measurements? 5. Summarize your data chart. Fill in the blanks in this chart. You started with 10 grams of iron, sulfur, and salt. After the experiment you had: Substance Mass Iron Salt Sulfur TOTAL 6. How did this experiment demonstrate the law of conservation of mass? What were some possible sources of error in your experiment? Calculate your percent error.