Oxidation Numbers
Oxidation State
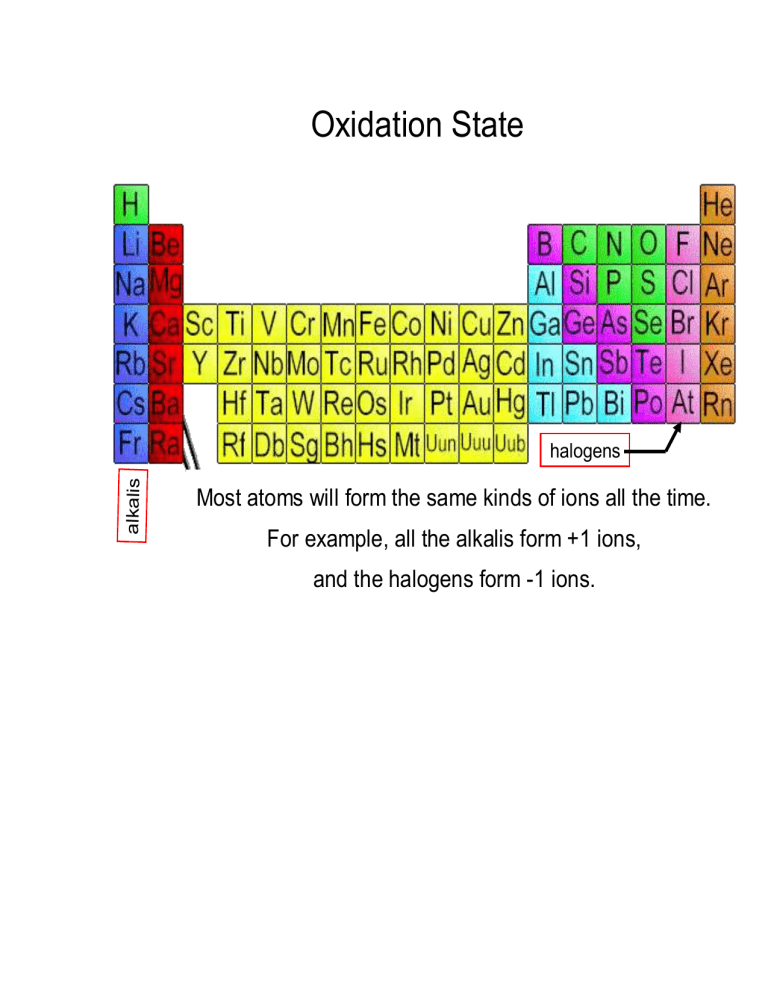
lka halogens
Most atoms will form the same kinds of ions all the time.
For example, all the alkalis form +1 ions, and the halogens form -1 ions.
Oxidized and Reduced States
transition metals
The transition metals are more electronically complex.
They may form ions of various charges.
For example, iron (Fe) is found as +2 and +3 ions.
A transition metal cation with a higher charge is more oxidized than one of lower charge. That comes from the fact that materials with high proportions of Fe +3 /Fe +2 form in environments where oxygen is abundant.
The opposite is also true, and we call Fe +2 reduced iron.
Oxidation Numbers
Related to the concept of valency is that of oxidation state. This is the number of electrons lost or gained by an atom of an element in a compound or ion treating all the bonds as ionic, i.e. assigning the electrons to the more electronegative element of the bond. Oxidation states of atoms which have ‘gained’ electrons are negative and atoms which have ‘lost’ electrons have positive oxidation states. In fact, in compounds other than ionic, while whole electrons have not been transferred, electron density has actually been
transferred towards the electronegative atoms.
Oxidation numbers of an atom in a molecule of a compound can be predicted using the following rules.
1. The sum of the oxidation numbers in a neutral compound must be zero and must add to give the overall charge if the species is ionic. E.g. NaCl
2. An atom of a free element has an oxidation state of zero.
E.g. Na, O
2
3. The oxidation state of the alkali metals in compounds is always +1 and similarly the alkaline earth metals in compounds always have oxidation state of +2. e.g. Na in NaCl
e.g. Mg in MgO
4. The oxidation state of hydrogen in most compounds is
+1. There is one group of compounds which is an important exception. In the metal hydrides, hydrogen has an oxidation state of -1 because hydrogen is more
electronegative than the metal element and is thus assigned the bonding electrons when determining oxidation states. e.g.NaH
5. The oxidation state of oxygen in most compounds is -2. The compounds OF
2
and the peroxides and superoxides are important exceptions. In oxygen difluoride,
OF
2
, the oxygen atom is bonded to the only other element which is more electronegative than it.
Therefore, the fluorine is assigned the bonding electrons when determining oxidation
states, giving oxygen an oxidation state of +2. In peroxides, each oxygen atom is bonded to another oxygen atom and to another more electropositive element. Since the two oxygen atoms have the same electronegativity value neither can obtain electrons from the other so bonding electrons can only be assigned to each oxygen from the electropositive atom, giving oxygen in peroxides an oxidation state of -1. p.190 14.5
What are the valencies and oxidation states of carbon in each of the compounds, carbon dioxide, methane, methanol and methanal?
The valency of carbon in each case is 4 since its four valence electrons are shared in each case. However, its oxidation number in carbon dioxide is +4 having bonded with two electronegative oxygens. In methane the oxidation number of carbon is -4 having bonded with more electropositive hydrogen. In methanol the oxidation number of carbon is -2, where carbon is bonded to more electronegative
oxygen and more electropositive hydrogen. Comparing the oxidation numbers of the carbon in methane with the carbon in methanol shows that electron density has been drawn away from the carbon by the more electronegative oxygen atom.
In methanal the oxidation number of carbon is zero, where carbon is bonded to more electronegative oxygen and more electropositive hydrogen again but now even more electron density has been drawn away from the carbon atom compared with methanol. The
carbon atom is losing electrons as it is oxidised from methanol to methanal.
Thus, valency gives the number of electrons involved in bonding but oxidation numbers provide
information about their distribution.
Oxidation numbers also provide information about oxidation and reduction of species in redox reactions. Since oxidation is defined as loss of electrons and reduction as gain of electrons, an increase in the oxidation number of an element indicates that it has been oxidised and a decrease in
oxidation number indicates reduction. For example, in the biological oxidation of glucose in respiration the carbon undergoes an increase in oxidation number from zero in glucose to +4 in carbon dioxide, indicating oxidation of the carbon. Is it evident?
C
6
H
12
O
6
+ 6O
2
→ 6CO
2
+ 6H
2
O
**Balancing Chemical Equations
Balanced chemical equations give the formulae for the reacting chemicals on the left and the
formulae of the products of the reaction on the right.
Additionally, the correct ratio of moles of each of these substances to one another needed for complete reaction is given.
In a balanced equation there are equal numbers of each type of atom on both sides of the equation.
This must be so since atoms cannot be inter converted in a chemical reaction and neither can they be destroyed or created so there must be the same number of each
type at the beginning and end of a chemical reaction.
Sometimes it is helpful to write only part of a chemical equation.
For example, in redox reactions some ions do not participate in the oxidation or reduction processes.
These are called spectator ions and it is sometimes helpful to write the chemical reaction omitting the spectator ions. The remaining ionic reactant and product species in the balanced equation must balance (i) in terms of atoms, (ii) in terms of
charge, and (iii) in terms of electron transfer.
For example, the reaction between potassium manganate(VII) and disodium ethanedioate under acidic conditions is represented by the following balanced equation.
2KMnO
4
+ 5(COONa)
2
+ 8H
2
SO
4
→ 2MnSO
4
+ 5Na
2
SO
4
+ K
2
SO
4
+ 8H
2
O + 10CO
2
Omitting the spectator ions, none of which undergoes an oxidation number change, gives
2MnO
4
– + 5(COO)
2
2– + 16H
8H
2
O + 10CO
2
+ → 2Mn 2+ +
Check no. of atoms of each type
Check total charge on left and right
(How many?)
Inspection of this balanced ionic equation shows that (i) there is the same number of each type of atom on the left and on the right of the equation, (ii) the total charge on all species on the left is +4 and the total charge on all species on the right is +4, and (iii) the change in oxidation number of both manganese atoms is -10 and the change in oxidation number of the ten carbon atoms is +10 so the equation is also balanced in terms
of electron transfer in the oxidation and reduction steps. It may be helpful to break down this ionic equation further to give the separate oxidation and reduction steps, or halfequations.
MnO
4
– + 8H + + 5e – → Mn 2+ + 4H
2
O
(COO)
2
2– → 2CO
2
+ 2e –
Note that each of these halfreactions is itself balanced in terms of (i) atoms and (ii) charge. Note also that in order for the charge transfers to
balance in the redox reaction the manganese half-reaction must occur twice while the carbon half-reaction occurs five times.
Worked Example p.188
Do 14.8 p.190
(a)






