Fluorescence Microscopy in Caenorhabditis elegans
advertisement

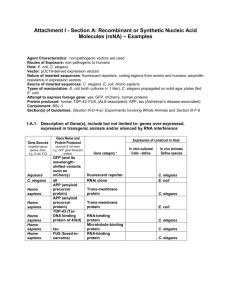
Fluorescence Microscopy in Caenorhabditis elegans NOTES and PROTOCOLS Marta Kostrouchová and Zdenek Kostrouch Caenorhabditis elegans – A powerful model organism of molecular biology Why to work on model organisms: Each organism - each individual is unique. In this line, we have to be aware that each patient is unique and medicine that will be shaped for individually adopted therapeutic approaches will continue to gain prevalence in the future. On the other hand, mechanisms that constitute the basis of individual metabolic, developmental and disease-linked states of individual organisms are to some extent shared. The closer the individual organisms are related, the more similar are the regulatory mechanisms that form the basis of the metabolism, development, reaction to stress and disease causing factors. These relations are valid not only for organisms of the same biological species but also for organisms in the whole domain of living organisms or cellular life (Cytota). Contemporary medicine is a biological discipline and in reverse, medicine is an important part of contemporary biology. The mechanisms that constitute existence of all species are connected by their evolutionary history, and reflect the similarities and differences between species. In order to understand the structural and regulatory mechanisms, we have to be aware of the context on which these mechanisms perform their biological roles. This means that biology of any system needs to be done in a complex way as a complex biology of the given species. Nevertheless, for many reasons, technical as well as ethical, some approaches are unthinkable on humans, vertebrates or a particular species. This is another reason, why biological research has to be done on certain species, which are called model organisms. Biology of Caenorhabditis elegans was elaborated to a very detailed and further dynamically developing complex biology of this specific organism and contributed to large number of critical discoveries, including developmental regulatory pathways, apoptosis, regulation by RNA interference and many other. The experimental tools and open informatics of this research system were enlarged for other experimental systems including human biology and medicine. Since many tools are shared between the C. elegans system and human biology and medicine, it may be inspirational and instructive for medical students to have a closer look on methods of the C. elegans research system and their link to medicine. Techniques and literature are freely accessible on WWW: 1/ Wormbase http://www.wormbase.org/ 2/ individual webpages e.g. http://hymanlab.mpi-cbg.de/hyman_lab/methods/c_elegans/techniques_fluo.shtml Lesson I: C. elegans biology. Handling worms in the laboratory. Points that will be discussed: Caenorhabditis elegans – an excellent model organism 1. It is an 1 mm long nematode, lives in soil and or water 2. Why we study model organisms 3. Several examples of model organisms 4. What we study in model organisms 5. C. elegans was introduced by Sydney Brenner in 1975 as a simple multicellular organism for genetic and behavioral studies and for study of the nervous system 6. It belongs to the phylum of nematodes, has a simple body, its body is transparent 7. They have fixed number of somatic cells, cell divisions are conserved, hermaphrodites 959, males 1031 8. It is easy to grow it in the laboratory, Petri dish, OP50, lives longer at lower temperature and with less food 9. It has a short life span, develops during 3 days from embryos to adults, reproduces for 3-4 days and lives 2 more weeks 10. It has many progeny – 300 (good genetic model), two sexes - hermaphrodites and males, hermaphrodites reproduce by self fertilization and by crossing with males 11. It is a very good experimental model – the first multi-cellular organism with sequenced genome (1998), 100Mbp and 22 000 genes x human 3000 Mbp and 22-25 000 genes 12. C. e. genes: -an average of five introns - exons comprise 27% of the genome. - 42% of predicted protein products match those of organisms in other phyla - the non-coding RNAs include: dispersed transfer RNA genes, tRNA- derived pseudogenes, spliceosomal RNA genes, and ribosomal RNA genes. the chromosomes have a GC content of 36% and have no localized centromeres. 13. Genetics: - forward – classical (from mutation to the gene) - reverse (from a gene to its loss of function phenotype) (by mutagenesis, RNA interference) 14. Preparation of transgenic organisms is easy - transcriptional fusion genes with gene, which codes for green fluorescent protein - translational fusion gene 15. RNA interference (A. Fire and G. Mello) – posttranscriptional effect 16. Communication – WormBase (http://www.wormbase.org/) Fig. 1 A scheme of an adult hermaphrodite: Fig. 2 A schema of gonads in hermaphrodite: The reproduction system: Fig. 3. An example of regulatory cascade discovered in C. elegans - Apoptosis: Note. Figures 1 – 3 are reproduced from the open source of Wormbase (http://www.wormbase.org/). Practical demonstration: Complex informatics of C. elegans – The C. elegans server and The Wormbase (http://www.wormbase.org/). Lesson II: Studying gene expression in living intact organisms Transgenic organisms - their preparation and detection 1. GFP Genes are tagged with the gene for green fluorescent protein from Aequorea Victoria (medusa - jelly fish). It produces a fluorescent product, when expressed in prokaryotic (E. coli) or eukaryotic (C. elegans) cells. Because exogenous substances and cofactors are not required for this fluorescence, GFP expression can be used to monitor gene expression and protein localization in living organisms. In Aequorea a light is produced when calcium binds to the photo protein aequorin. It produces a blue light. Green light is a result of a second protein that derives its excitation energy from aequorin, the GFP. Purified GFP: - has 238 amino acids - absorbs blue light maximally at 395nm - emits green light at 509nm - the fluorescence is very stable, with no photo bleaching The expression of the protein in C. elegans starts when natural expression starts, - it lasts 10 minutes when illuminated with 450 to 490 nm light - it does not interfere with cell growth and function 2. Reporter gene fusions A. Transcriptional reporters consist of - a promoter fragment, a region upstream of a gene of interest driving GFP (in frame fusion); - The length of the promoter is for genes in C. elegans usually a few kb immediately upstream of the start codon (1.5 – 2) - ATG from the first methionine should be included B. Translational reporters consist of - an entire genomic locus of a gene: 5’ upstream region , all gDNA and 3’UTR - GFP can be inserted at any point in the open reading frame, not to disrupt protein function or topology Sometimes the regulatory sequences can be found within introns, if they are very large, or at long distances from the gene of interest 3. Multicolor GFP expression systems The existence of GFP variants with non-overlapping emission spectra: Cyan-FP Yellow-FP Allows the possibility for multi-color labeling of C. elegans cells. 4. Artifacts - Natural auto-fluorescence of gut granules and nucleoli of hypodermal cells. - Non specific fluorescence in posterior gut cells. 5. Co - injection markers: Rol-6 (su1006): is a dominant allele allowing easy selection of transgenic lines 6. Is this real expression what we see? The expression pattern has to be conformed by preparation of several transgenic lines, by other methods as by in situ hybridization, by antibody staining etc. Fig. 4 Caenorhabditis elegans at laboratory conditions. A – Mixed stages of C. elegans on an agar plate with bacteria E. coli. B – A hermaphrodite with embryos developing inside the uterus. Shead cuticle of some other worm is visible on the right. C – A two cell stage embryo. D – An embryo in a comma stage. E – An embryo in two fold stage. F – A larva in L4 stage. G – A L3 larva expressing nhr-153::gfp in pharynx and in intestinal cells.